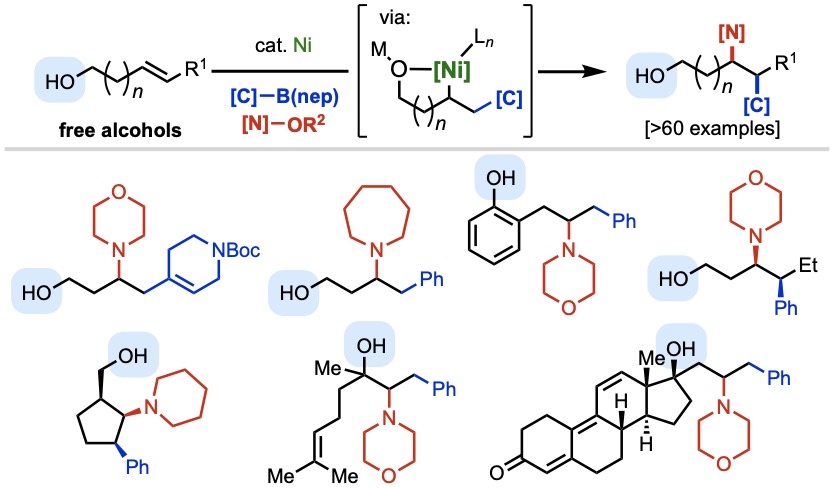
In a breakthrough report that pushes the frontier of practical utility in nickel-catalyzed alkene functionalization, Taeho, Nana, and collaborators at Bristol Myers Squibb have discovered a method to carry out selective 1,2-carboamination of free alkenyl alcohols with aryl/alkenylboronic acids and nitrogen electrophiles. Key to the success of this transformations is the careful design of the activating group for the N–O electrophile, which promotes the desired three-component process and suppresses side reactions. The reaction enables rapid synthesis of alkyl-amine-containing bioactive small molecules and late-stage modification of natural products. Bravo on this one, folks!
For a link to pre-print, click here: https://chemrxiv.org/articles/preprint/Nickel-Catalyzed_1_2-Carboamination_of_Alkenyl_Alcohols/14195426