BIBLIOMETRICS
• Google Scholar
• ResearcherID
• ResearchNet
PRE-PRINTS
PEER-REVIEWED PUBLICATIONS
131. Zhao, H.; Ravn, A. K.; Haibach, M. C.; Engle, K. M.; Johansson Seechurn, C. C. C. C. “The Diversification of Pharmaceutical Manufacturing Processes: Taking the Plunge into the Non-PGM Catalysis Pool,” ACS Catal. 2024, In Press.
• Pre-print uploaded to ChemRxiv on March 11, 2024 (DOI: 10.26434/chemrxiv-2024-twzr2)
130. Rubel, C. Z.; Ravn, A. K.; Ho, H. C.; Yang, S.; Li, Z.-Q.; Engle, K. M.; Vantourout, J. C. “Stereodiverent, Kinetically Controlled Isomerization of Terminal Alkenes via Nickel Catalysis,”Angew. Chem. 2024, 136, e202320081; Angew. Chem. Int. Ed. 2024, 63, e202320081.
• Pre-print uploaded to ChemRxiv on December 27, 2023 (DOI: 10.26434/chemrxiv-2022-x8ssk-v2)

129. Laudadio, G.; Neigenfind, P.; Péter, A.; Rubel, C. Z.; Emmanuel, M. A.; Oderinde, M. S.; El-Hayek Ewing, T.; Palkowitz, M. D.; Sloane, J. L.; Gillman, K. W.; Ridge, D.; Mandler, M. D.; Bolduc, P.; Nicastri, M.; Zhang, B.; Clementson, S.; Peterson, N. N.; Martín-Gago, P; Mykhailiuk, P.; Engle, K. M.; Baran P. S. “Ni-Electrocatalytic Decarboxylative Arylation to Access Quaternary Centers,” Angew. Chem. 2024, 136, e202314617; Angew. Chem. Int. Ed. 2024, 63, e202314617.
• Pre-print uploaded to ChemRxiv on September 4, 2023 (DOI: 10.26434/chemrxiv-2023-srlm6-v2)

128. Rubel, C. Z.; He, W.-J.; Wisniewski, S. R.; Engle, K. M. “Benchtop Nickel Catalysis Invigorated by Electron-Deficient Diene Ligands,” Acc. Chem. Res. 2024, 57, 312–326.
• Part of the special issue, Cross-Coupling with First-Row Metals
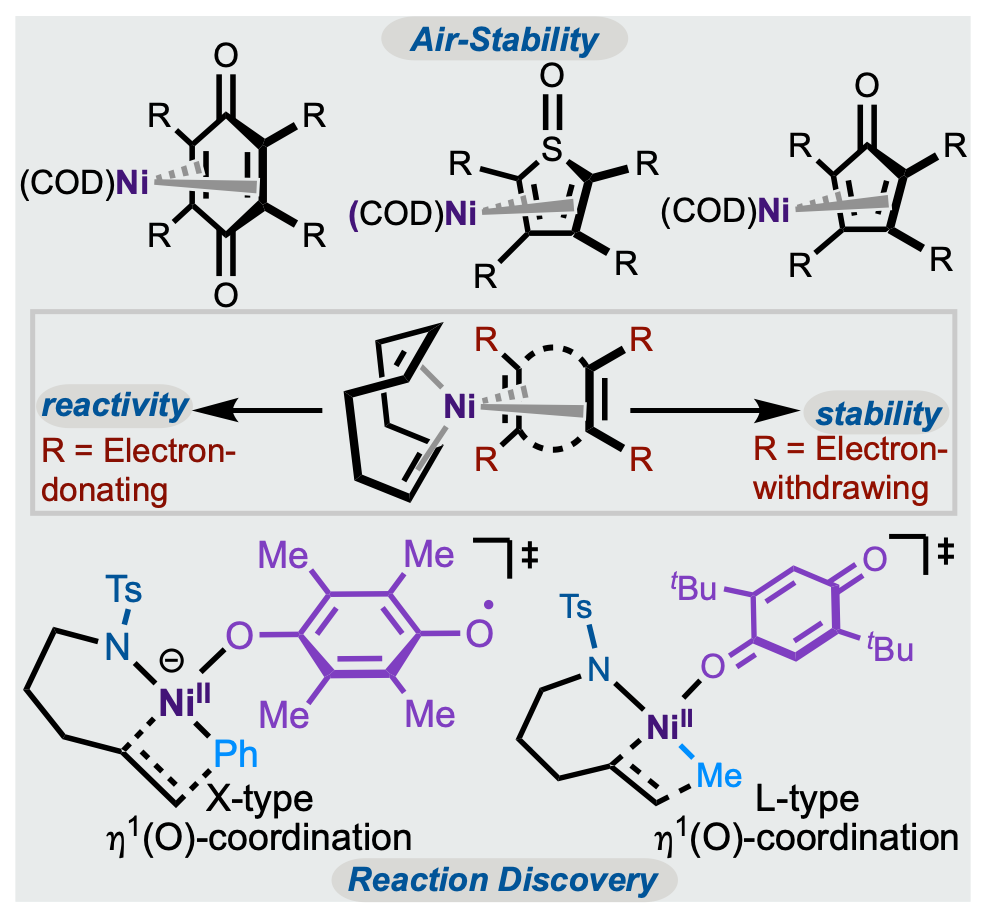
126. Rubel, C. Z.; Cao, Y.; El-Hayek Ewing, T.; Laudadio, G.; Beutner, G.; Wu, X.; Wisniewski, S. R.; Baran, P. S.; Vantourout, J. C.; Engle, K. M. “Electroreductive Synthesis of Ni(0) Complexes,” Angew. Chem. 2024, 136, e202311557; Angew. Chem. Int. Ed. 2024, 63, e202311557.
• Pre-print uploaded to ChemRxiv on April 4, 2023 (DOI: 10.26434/chemrxiv-2023-sgnl1)
• Selected as a Hot Paper
• Highlighted as an item of interest in OPR&D


124. Cao, Y.; Li, Z.-Q.; Engle, K. M. “Ni-Catalyzed 1,2-Diarylation of Unactivated Alkenes Directed by Diverse Azaheterocycles,” Tetrahedron Lett. 2023, 132, 154764.
• Special Issue for Bill Morandi’s 2023 Tetrahedron Young Investigator Award

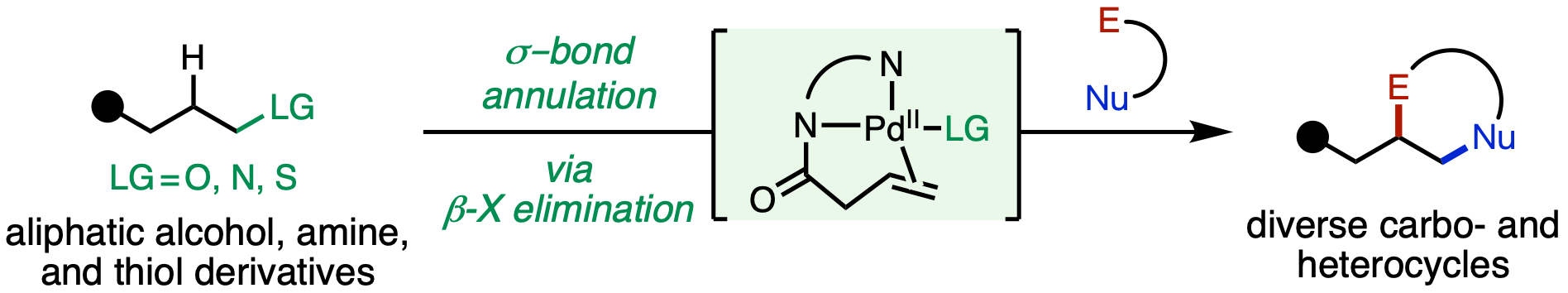
123. Ni, H.-Q.; Dai, J.-C.; Yang, S.; Loach, R. P.; Chuba, M. D.; McAlpine, I. J.; Engle, K. M. “Catalytic σ-Bond Annulation with Ambiphilic Organohalides Enabled by β-X Elimination,” Angew. Chem. 2023, 136, e202306581; Angew. Chem. Int. Ed. 2023, 62, e202306581.
• Pre-print uploaded to ChemRxiv on May 11, 2023 (DOI: 10.26434/chemrxiv-2023-qv5bh).
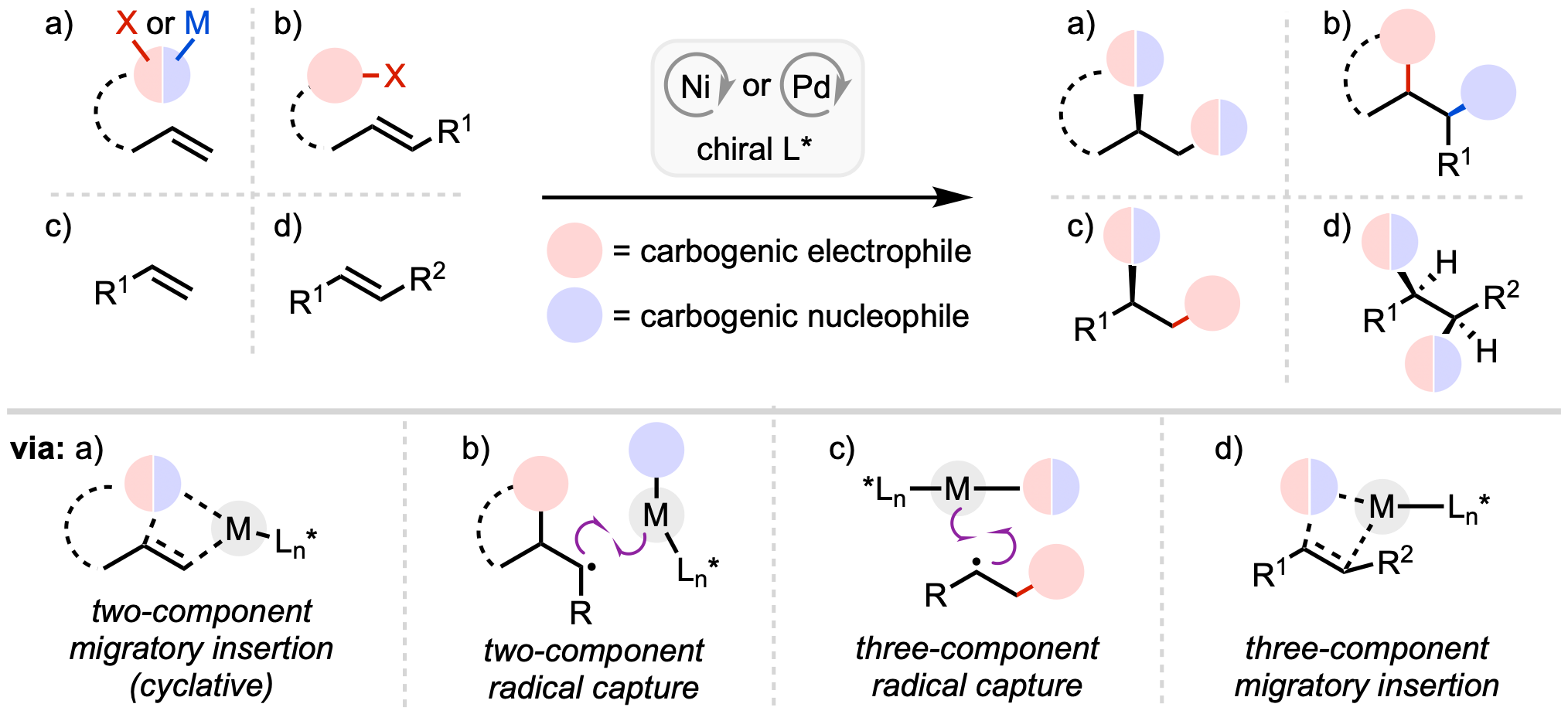
121. Ni, H.-Q.; Karunananda, M. K.; Zeng, T.; Yang, S.; Liu, Z.; Houk, K. N.; Liu, P.; Engle, K. M. “Redox-Paired Alkene Difunctionalization Enables Skeletally Divergent Synthesis,” J. Am. Chem. Soc. 2023, 145, 12351–12359.
• Pre-print uploaded to ChemRxiv on Dec 30, 2022 (DOI: 10.26434/chemrxiv-2022-jc6tc)

120. Simlandy, A. K.; Alturaifi, T. M.; Nguyen, J. M.; Oxtoby, L. J.; Wong, Q. N.; Chen, J. S.; Liu, P.; Engle, K. M. “Enantioselective Hydroalkenylation and Hydroalkynylation of Alkenes Enabled by a Transient Directing Group: Catalyst Generality through Rigidification,” Angew. Chem. 2023, 135, e202304013; Angew. Chem. Int. Ed. 2023, 62, e202304013.
• Pre-print uploaded to ChemRxiv on Jun 13, 2022 (DOI: 10.26434/chemrxiv-2022-h8w0k)

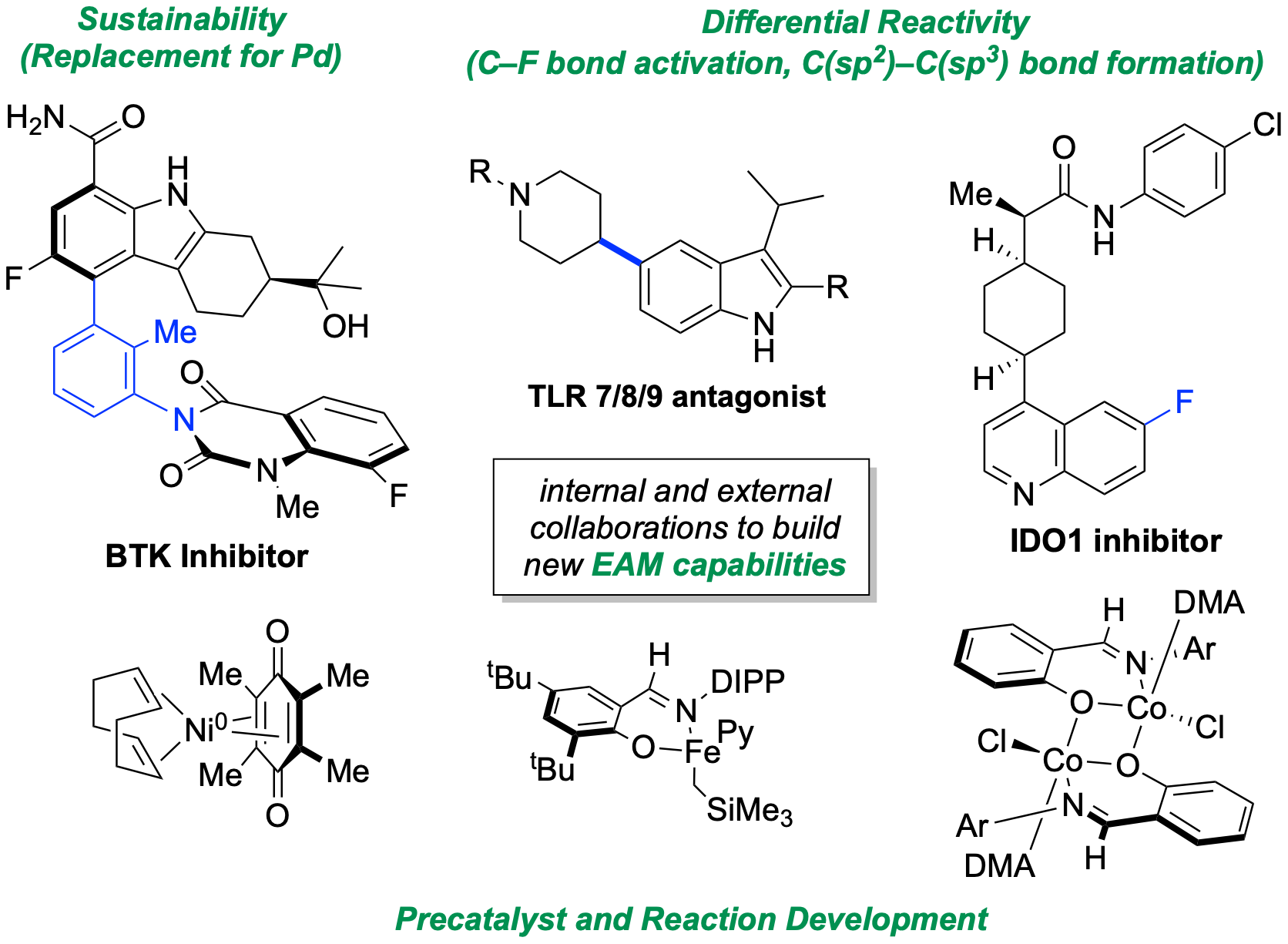
117. Tran, V. T.†; Kim, N.†; Rubel, C. Z.; Wu, X.; Kang, T.; Jankins, T. C.; Li, Z.-Q.; Joannou, M. V.; Ayers, S.; Gembicky, M.; Bailey, J.; Sturgell, E. J.; Sanchez, B. B.; Chen, J. S.; Lin, S.; Eastgate, M. D.; Wisniewski, S. R.; Engle, K. M. “Structurally Diverse Bench-Stable Nickel(0) Pre-Catalysts: A Practical Toolkit for In Situ Ligation Protocols,” Angew. Chem. 2023, 135, e202211794; Angew. Chem. Int. Ed. 2023, 62, e202211794. (†Authors contributed equally)
• Pre-print uploaded to ChemRxiv on Feb 11, 2022 (DOI: 10.26434/chemrxiv-2022-7zjvh)
• Highlighted in OPRD
• Highlighted in C&E News

116. Kang, T.; Fu, Y.; Matsuura, R.; Liu, A. L.; Jankins, T. C.; Rheingold, A. L.; Bailey, J. B.; Gembicky, M.; Liu, P.; Engle, K. M. “Synthesis and Characterization of Post-β-Carbon-Elimination Organopalladium Complexes,” Organometallics 2023, 42, 11–15.
• Pre-print uploaded to ChemRxiv on Oct 31, 2022 (DOI: 10.26434/chemrxiv-2022-8wbgn)

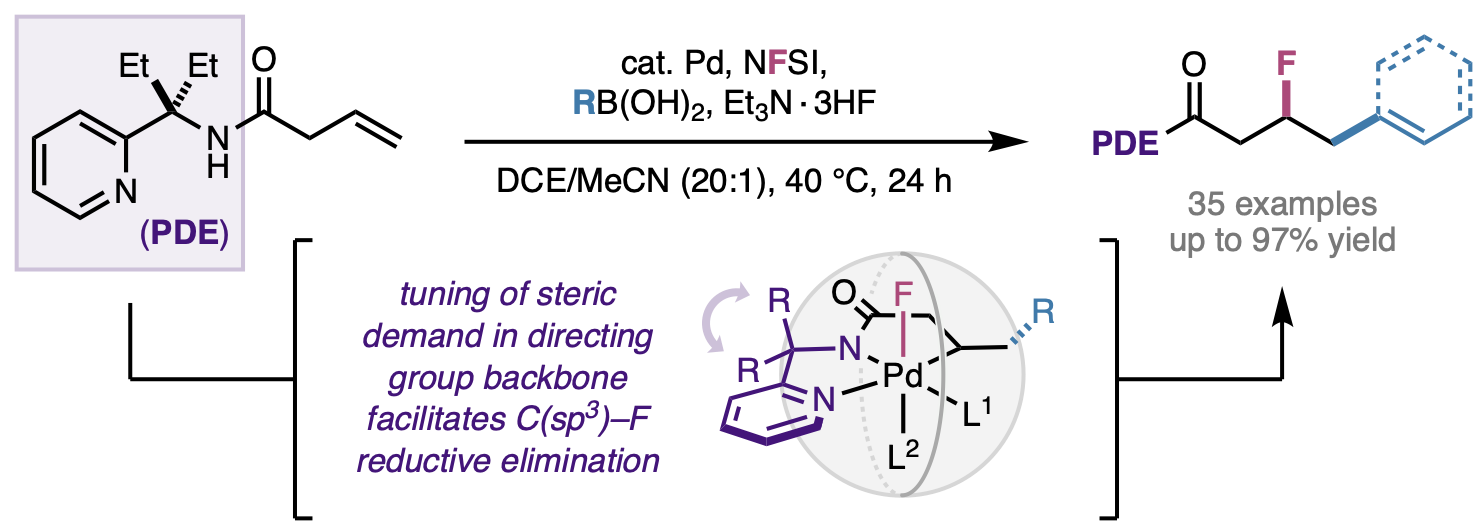
115. Liu, Z.; Oxtoby, L. J.; Sun, J.; Li, Z.-Q.; Kim, N.; Davies, G. H. M.; Engle, K. M. “A Sterically Tuned Directing Auxiliary Promotes Catalytic 1,2-Carbofluorination of Alkenyl Carbonyl Compounds,” Angew. Chem. 2023, 135, e202214153; Angew. Chem. Int. Ed. 2023, 62, e202214153.
• Pre-print uploaded to ChemRxiv on Apr 25, 2022 (DOI: 10.26434/chemrxiv-2022-t2zb4)
114. Simlandy, A. K.; Rodphon, W.; Alturaifi, T. M.; Mai, B. K.; Ni, H.-Q.; Gurak, J. A., Jr.; Liu, P.; Engle, K. M. “Catalytic Addition of Nitroalkanes to Unactivated Alkenes via Directed Carbopalladation,” ACS Catal. 2022, 12, 13755–13762.
• Pre-print uploaded to ChemRxiv on Feb 1, 2022 (DOI: 10.26434/chemrxiv-2022-1q5dw-v2)
• Highlighted in ChemistryViews
• Highlighted in Synfacts

113. Apolinar, O.†; Kang, T.†; Alturaifi, T. M.; Bedekar, P. G.; Rubel, C. Z.; Derosa, J.; Sanchez, B. B.; Wong, Q. N.; Sturgell, E. J.; Chen, J. S.; Wisniewski, S. R.; Liu, P.; Engle, K. M. “Three-Component Asymmetric Ni-Catalyzed 1,2-Dicarbofunctionalization of Unactivated Alkenes via Stereoselective Migratory Insertion,” J. Am. Chem. Soc. 2022, 144, 19337–19343. (†Authors contributed equally)
• Pre-print uploaded to ChemRxiv on Jun 24, 2022 (DOI: 10.26434/chemrxiv-2022-lvtjq)
• Highlighted in Synfacts

112. Liu, M.; Sun, J.†; Zhang, T.†; Ding, Y.; Han, Y.-Q.; Martín-Montero, R.; Lan, Y.; Shi, B.-F.; Engle, K. M. “Regio- and Stereoselective 1,2-Oxyhalogenation of Non-Conjugated Alkynes via Directed Nucleopalladation: Catalytic Access to Tetrasubstituted Alkenes,” Angew. Chem. 2022, 134, e202209099; Angew. Chem. Int. Ed. 2022, 61, e202209099. (†Authors contributed equally)
• Pre-print uploaded to ChemRxiv on Jun 26, 2022 (DOI: 10.26434/chemrxiv-2022-kd15t)

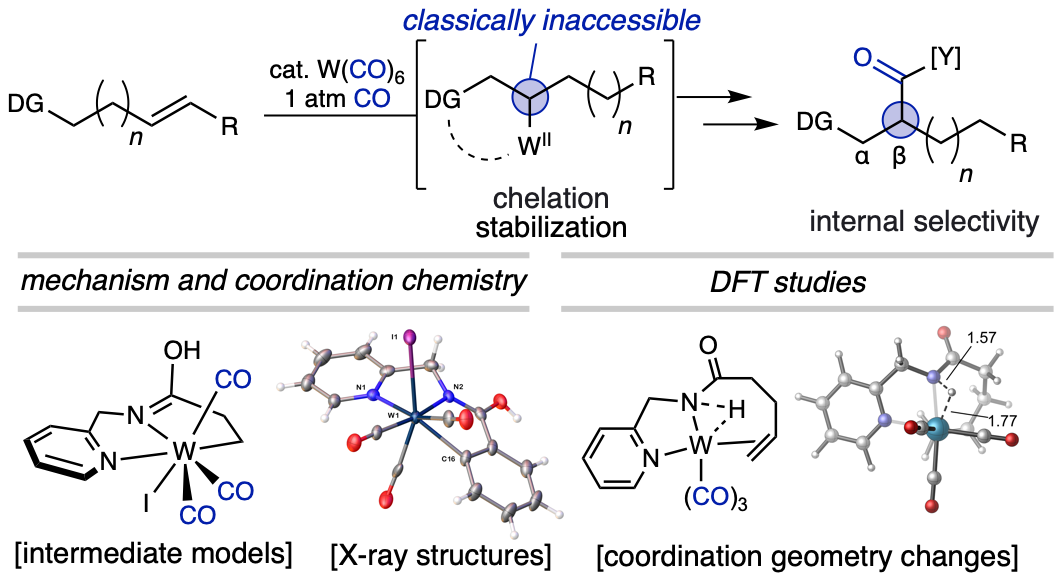
110. Jankins, T. C.; Bell, W. C.; Zhang, Y.; Qin, Z-.Y.; Gembicky, M.; Chen, J. S.; Liu, P.; Engle, K. M. “Low-Valent Tungsten Redox Catalysis Enables Controlled Isomerization and Cabonylative Functionalization of Alkenes,” Nat. Chem. 2022, 14, 632–639
• Pre-print uploaded to ChemRxiv on Apr 4, 2021 (DOI: 10.26434/chemrxiv.14362238)
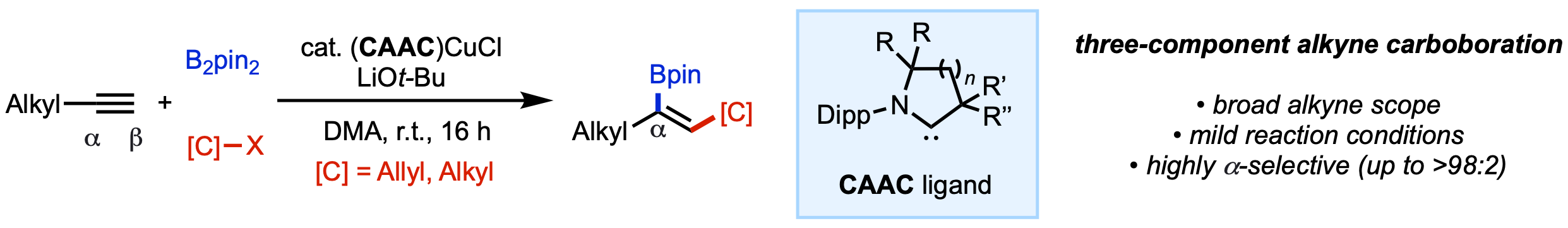
109. Gao, Y.; Kim, N.;† Mendoza, S. D.†; Yazdani, S.; Liu, M.; Kendrick, A., IV, Grotjahn, D. B.; Bertrand, G.; Jazzar, R.; Engle, K. M. “(CAAC)Copper Catalysis Enables Regioselective Three-Component Carboboration of Terminal Alkynes,” ACS Catal. 2022, 12, 7243–7247. (†Authors contributed equally).
• Pre-print uploaded to ChemRxiv on Feb 1, 2022 DOI: 10.26434/chemrxiv-2022-z4wg2
• Highlighted in ChemistryViews
108. Li, Z.-Q.; He, W.-J.; Ni, H.-Q.; Engle, K. M. “Directed, Nickel-Catalyzed 1,2-Alkylsulfenylation of Alkenyl Carbonyl Compounds,” Chem. Sci. 2022, 13, 6567–6572.
• Pre-print uploaded to ChemRxiv on Mar 18, 2022 (DOI: 10.26434/chemrxiv-2022-89l57
107. Liu, M.; Sun, J.; Erbay, T.; Ni, H.-Q.; Martín-Montero, R.; Liu, P.; Engle, K. M. “Pd(II)-Catalyzed C(alkenyl)–H Activation Facilitated by a Transient Directing Group,” Angew. Chem. Int. Ed. 2022, 61, e202203624, Angew. Chem. 2022, 134, e202203624.
• Pre-print uploaded to ChemRxiv on Mar 7, 2022 (DOI: 10.26434/chemrxiv-2022-d0zfn)
• Selected as a Hot Paper
• Highlighted in Synfacts
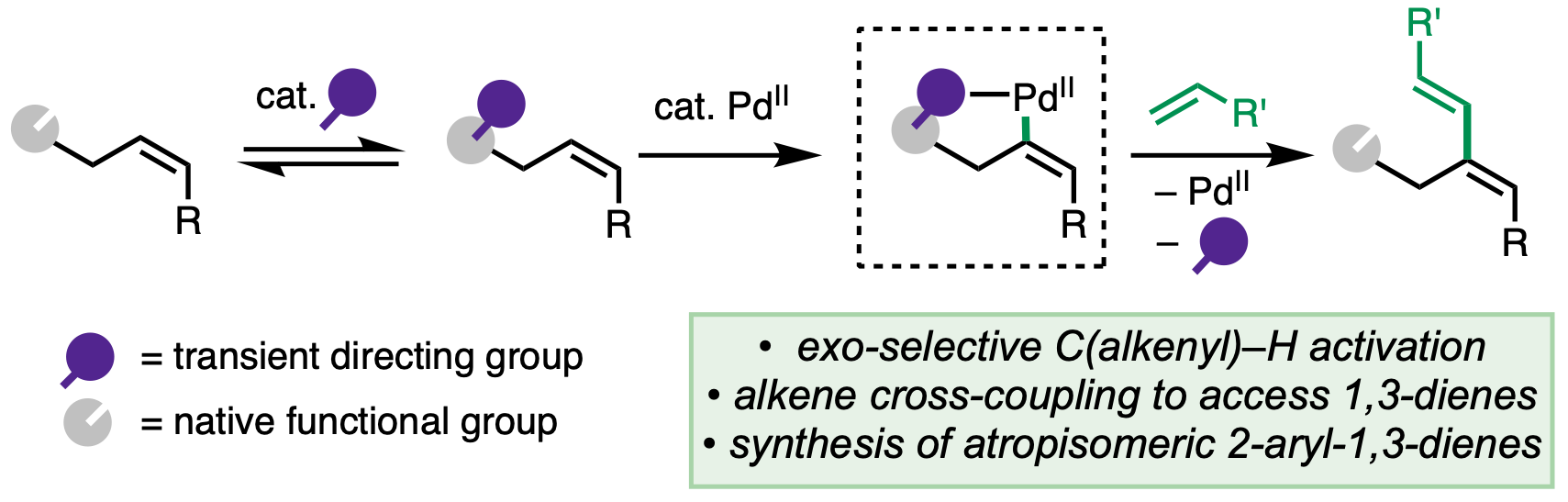
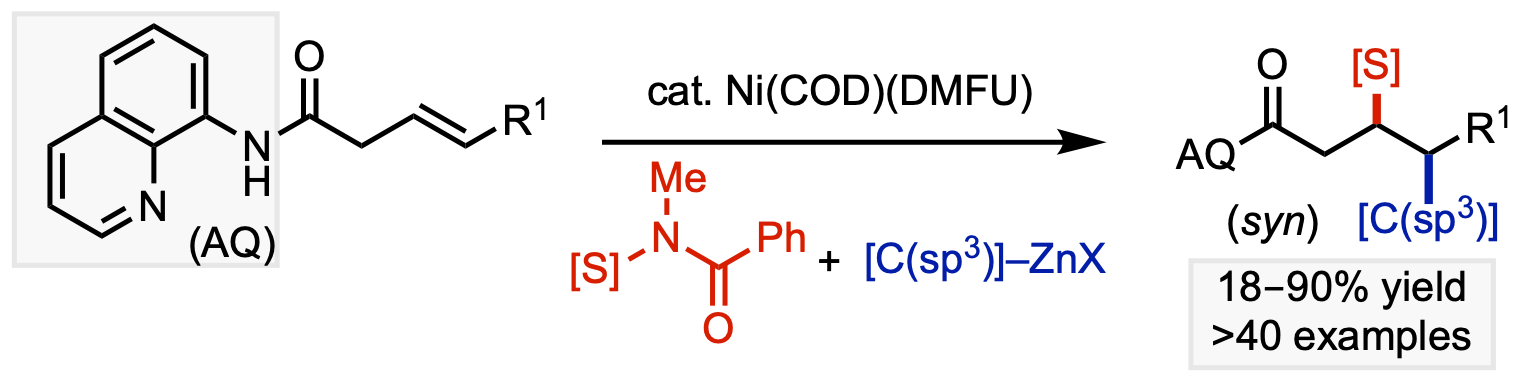
106. Li, Z.-Q.; Cao, Y.; Kang, T.; Engle, K. M. “Electrophilic Sulfur Reagent Design Enables Directed syn-Carbosulfenylation of Unactivated Alkenes,” J. Am. Chem. Soc. 2022, 144, 7189–7197.
• Pre-print uploaded to ChemRxiv on Dec 2, 2021 (DOI: 10.26434/chemrxiv-2021-bj205)
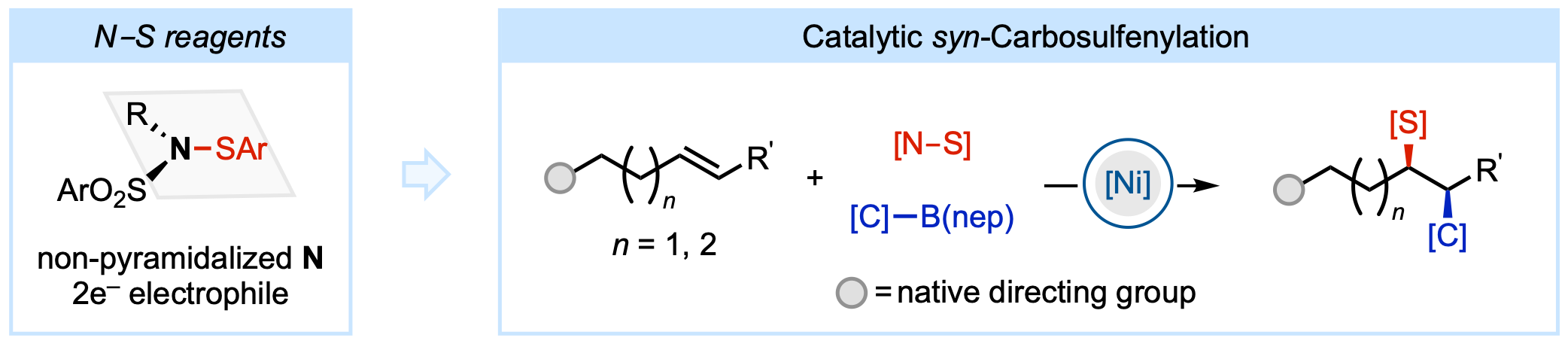
105. Kang, T.; González, J. M.; Li, Z.-Q.; Foo, K.; Cheng, P. T. W.; Engle, K. M. “Alkene Difunctionalization Directed by Free Amines: Diamine Synthesis via Nickel-Catalyzed 1,2-Carboamination,” ACS Catal 2022, 12, 3890–3896.
• Pre-print uploaded to ChemRxiv on Dec 9, 2021 (DOI: 10.26434/chemrxiv-2021-x6h4c)

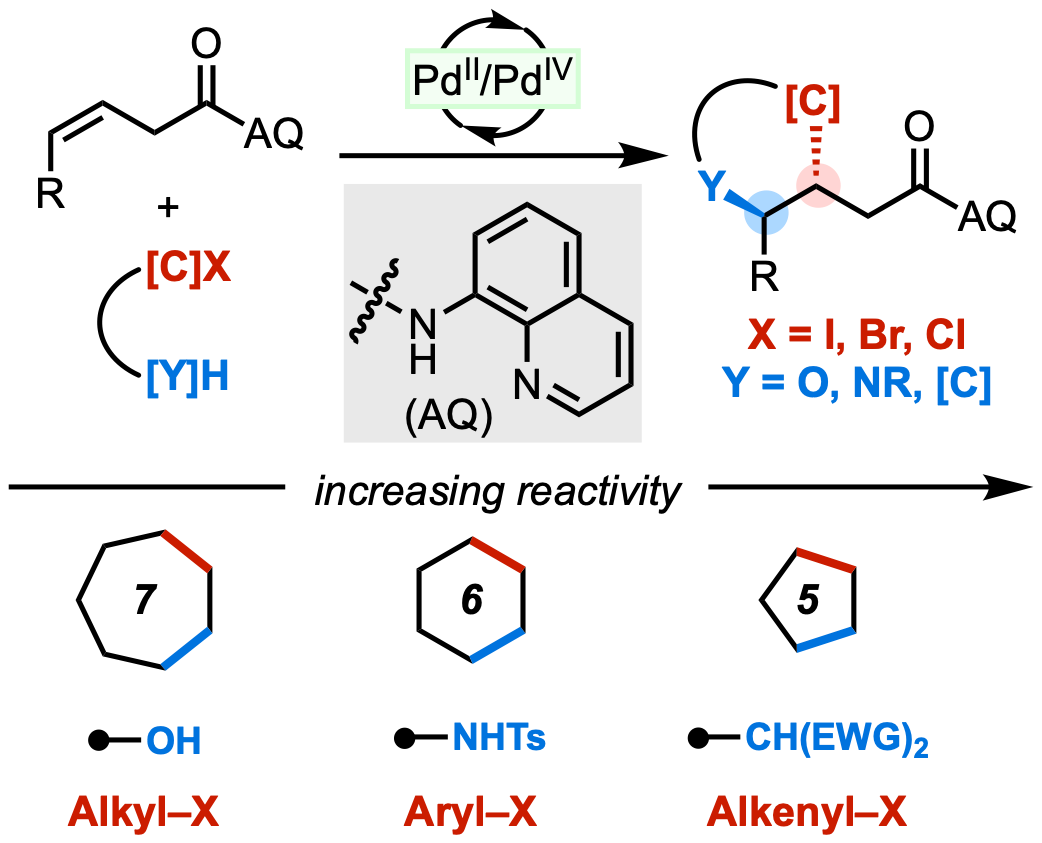
104. Ni, H.-Q.; Cooper, P. Yang, S.; Wang, F.; Sach, N.; Bedekar, P. G.; Donaldson, J. S.; Tran-Dubé, McAlpine, I. J.; Engle, K. M. “Mapping Ambiphile Reactivity Trends in the Anti-(Hetero)annulation of Non-conjugated Alkenes via Pd(II)/Pd(IV) Catalysis,” Angew. Chem. 2022, 134, e202114346; Angew. Chem. Int. Ed. 2022, 61, e202114346.
• Selected as a Hot Paper
• Pre-print uploaded to ChemRxiv on Oct 25, 2021 (Pre-print DOI: 10.33774/chemrxiv-2021-fzjx8)


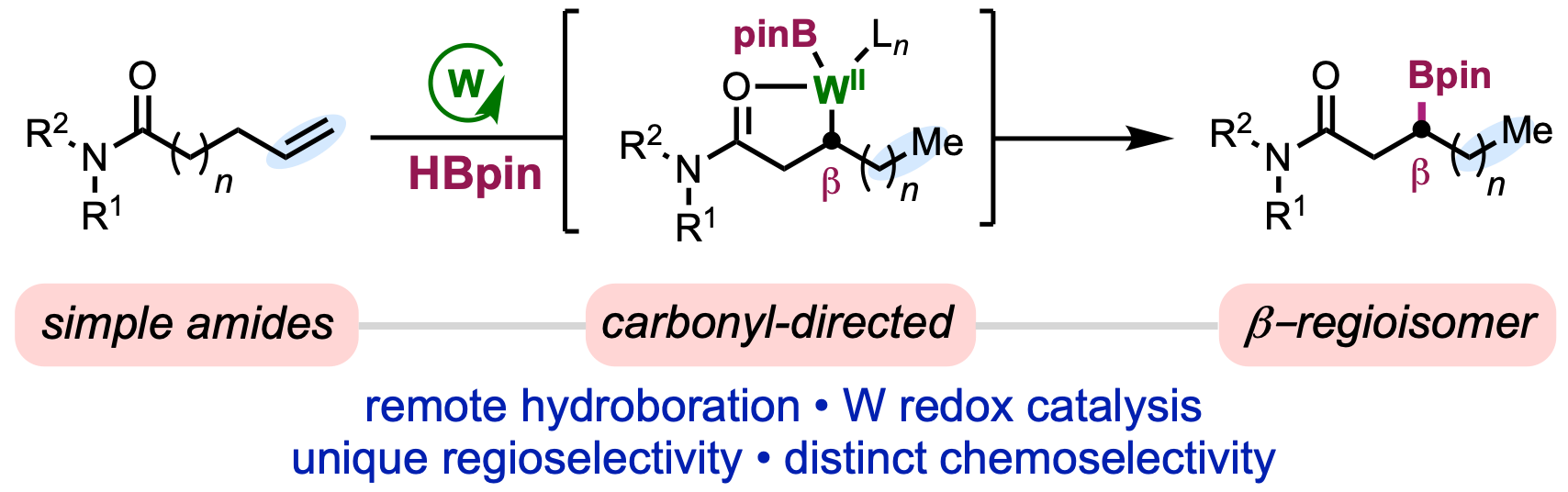
101. Jankins, T. C.†; Martin-Montero, R.†; Cooper, P.; Martin, R.; Engle, K. M. “Low-Valent Tungsten Catalysis Enables Site-Selective Isomerization–Hydroboration of Unactivated Alkenes,” J. Am. Chem. Soc. 2021, 143, 14981–14986. (†Authors contributed equally)
• Pre-print uploaded to ChemRxiv on July 12, 2021 (DOI: 10.33774/chemrxiv-2021-kn5xp)
100. Kang, T.; Kim, N.; Cheng, P. T.; Zhang, H.; Foo, K.; Engle, K. M. “Nickel-Catalyzed 1,2-Carboamination of Alkenyl Alcohols,” J. Am. Chem. Soc. 2021, 143, 13962–13970.
• Pre-print uploaded to ChemRxiv on March 16, 2021 (DOI: 10.26434/chemrxiv.14195426)

99. Li, Z.-Q.; Apolinar, O.; Deng, R.; Engle, K. M. “Directed Markovnikov Hydroarylation and Hydroalkenylation of Alkenes Under Nickel Catalysis,” Chem. Sci. 2021, 12, 11038–11044.
• Pre-print uploaded to ChemRxiv on May 25, 2021 (DOI: 10.26434/chemrxiv.14639778)
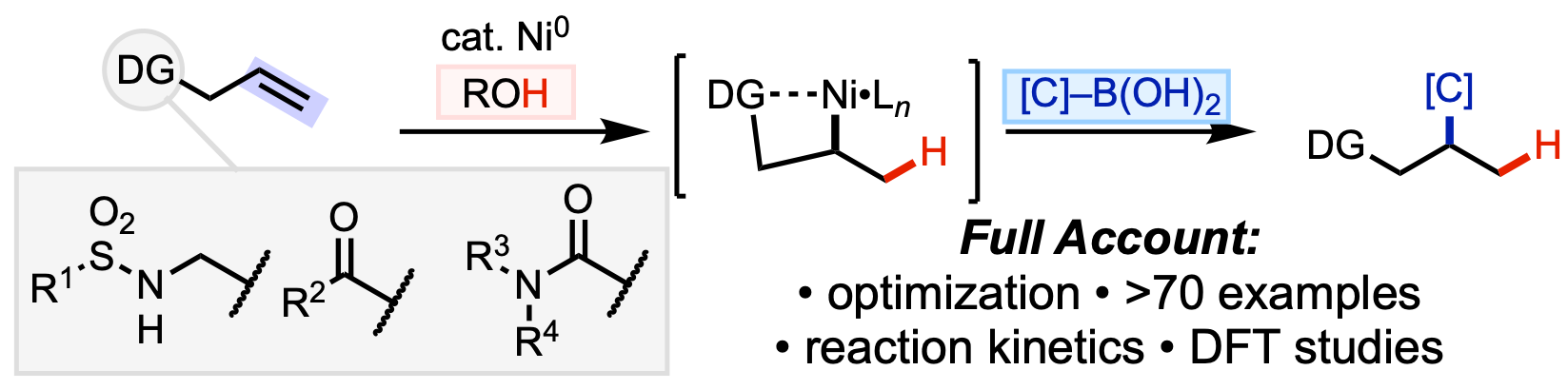
98. Ni, H.-Q.†; Cooper, P.†; Engle, K. M. “Recent Advances in Palladium-Catalyzed (Hetero)annulation of C=C Bonds with Ambiphilic Aryl (Pseudo)halides,” Chem. Commun. 2021, 57, 7610–7624.(†Authors contributed equally)
• Part of the 2021 Emerging Investigators Collection

97. Gao, Y.; Yazdani, S.; Kendrick, A., IV; Junor, G. P.; Kang, T.; Grotjahn, D. B.; Bertrand, G.; Jazzar, R.; Engle, K. M. “Cyclic(Alkyl)(Amino)Carbene Ligands Enable Cu-Catalyzed Markovnikov Protoborylation and Protosilylation of Terminal Alkynes: A Versatile Portal to Functionalized Alkenes,” Angew. Chem. Int. Ed. 2021, 60, 19871–19878; Angew. Chem. 2021, 133, 20024–20031.
• Pre-print uploaded to ChemRxiv on Apr. 12, 2021 (DOI: 10.26434/chemrxiv.14368607)

96. Kleinmans, R.†; Apolinar, O.†; Derosa, J.; Karunananda, M. K.; Li, Z.-Q.; Tran, V. T.; Wisniewski, S. R.; Engle, K. M. “Nickel-Catalyzed 1,2-Diarylation of Alkenyl Ketones: A Comparative Study of Carbonyl-Directed Reaction Systems,” Org. Lett. 2021, 23, 5311–5316. (†Authors contributed equally)
• Pre-print uploaded to ChemRxiv on Mar. 3, 2021 (DOI: 10.26434/chemrxiv.14150174)

95. Ni, H.-Q.; Li, Z.-Q.; Tran, V. T.; Engle, K. M. “Modular Synthesis of Non-Conjugated N-(Quinolin-8-yl) Alkenyl Amides via Cross-Metathesis,” Tetrahedron 2021, 93, 132279.
• Special Issue for Guangbin Dong’s 2021 Tetrahedron Young Investigator Award
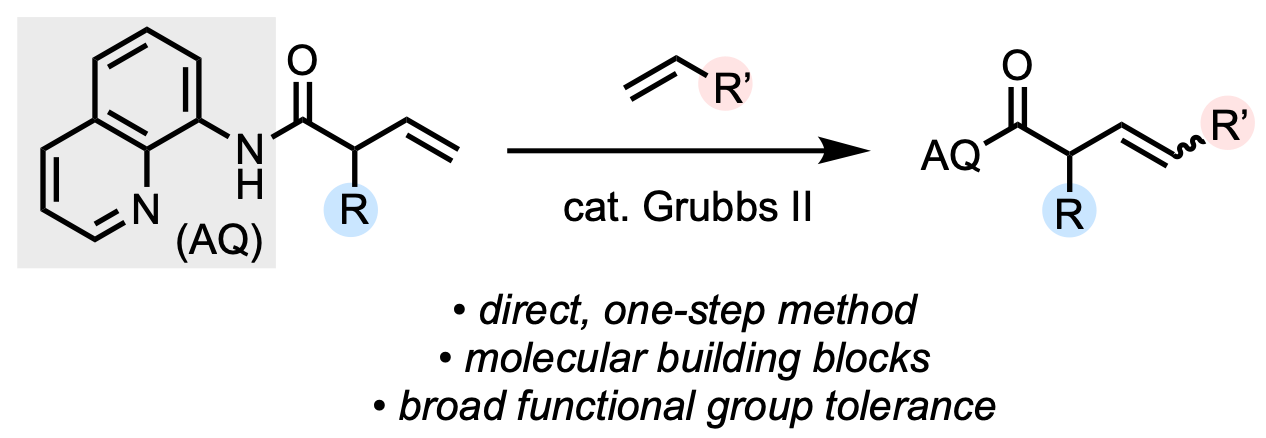
94. Liu, Z.; Oxtoby, L. J.; Liu, M.; Li, Z.-Q.; Tran, V. T.; Gao, Y.; Engle, K. M. “A Transient Directing Group Strategy Enables Enantioselective Multicomponent Organofluorine Synthesis,” J. Am. Chem. Soc. 2021, 143, 8962–8969.
• Pre-print uploaded to ChemRxiv on Jan. 7, 2021 (DOI: 10.26434/chemrxiv.13537283)
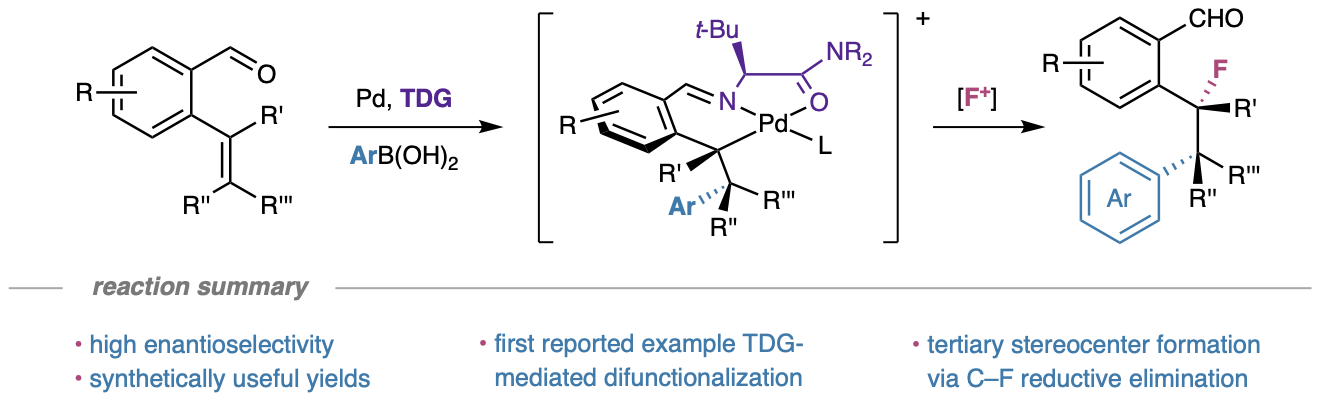
93. Romine, A. M.; Demer, M. J.; Gembicky, M.; Rheingold, A. L.; Engle, K. M. “Ligand Rearrangement Leads to Tetrahydrothiophene-Functionalized N,S-Heterocyclic Carbene Palladium(II) Complexes,” Organometallics 2021, 40, 2311–2319.
• Pre-print uploaded to ChemRxiv on Jan. 25, 2021 (DOI: 10.26434/chemrxiv.13633301)
• Part of the special Issue on “Organometallic Solutions to Challenges in Cross-Coupling”

92. Matsuura, R.; Karunananda, M. K.; Liu, M.; Nguyen, N.; Blackmond, D. G.; Engle, K. M. “Mechanistic Studies of Pd(II)-Catalyzed E/Z Isomerization of Unactivated Alkenes: Evidence for a Monometallic Nucleopalladation Pathway,” ACS Catal. 2021, 11, 4239–4246.
• Pre-print uploaded to ChemRxiv on Nov. 6, 2020 (DOI: 10.26434/chemrxiv.13194932)

91. Liu, M.; Tang, T.; Apolinar, O.; Matsuura, R.; Bussaca, C. A.; Qu, B.; Fandrick, D. R.; Zatolochnaya, O. V.; Senanayake, C. H.; Song, J. J.; Engle, K. M. “Atom-Economical Cross-Coupling of Internal and Terminal Alkyne to Access 1,3-Enynes,” J. Am. Chem. Soc. 2021, 143, 3881–3888.
• Pre-print uploaded to ChemRxiv on Oct. 15, 2020 (DOI: 10.26434/chemrxiv.13083419)
• Highlighted in ChemistryViews.

90. Kim, N.; Tran, V. T.; Apolinar, O.; Wisniewski, S. R.; Eastgate, M. D.; Engle, K. M. “Ni(COD)(DMFU): A Heteroleptic 16-Electron Precatalyst for 1,2-Diarylation of Alkenes,” Synlett 2021, 32, 1570–1754.
• Part of the special cluster in nickel catalysis organized by Profs. Rubén Martin and Gary Molander

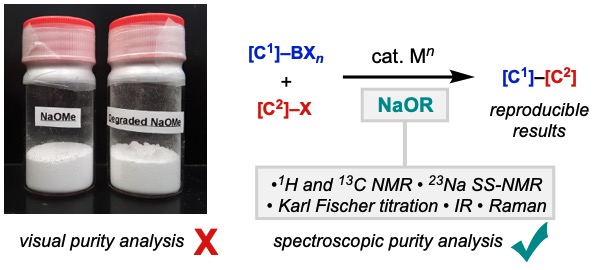
89. Wethman, R.; Derosa, J. Tran, V. T.; Kang, T.; Apolinar, O.; Abraham, A.; Kleinmans, R.; Wisniewski, S. R.; Coombs, J . R.; Engle, K. M. “An Under-Appreciated Source of Reproducibility Issues in Cross-Coupling: Solid-State Decomposition of Primary Sodium Alkoxides in Air,” ACS Catal. 2021, 11, 502–508.
• Pre-print uploaded to ChemRxiv on Aug. 17, 2020 (DOI: 10.26434/chemrxiv.12818234)
• Featured in Chemistry World
88. Ni, H.-Q.; Kevlishvili, I.; Bedekar, P. G.; Barber, J. S.; Yang, S.; Tran-Dubé, M.; Romine, A. M.; Lu, H.-X.; McAlpine, I.; Liu, P.; Engle, K. M. “Anti-Selective [3+2] (Hetero)annulation of Non-Conjugated Alkenes via Directed Nucleopalladation,” Nat. Commun. 2020, 11, 6432.
• Pre-print uploaded to ChemRxiv on Jun. 19, 2020 (DOI: 10.26434/chemrxiv.12510038)
• Highlight in Synform
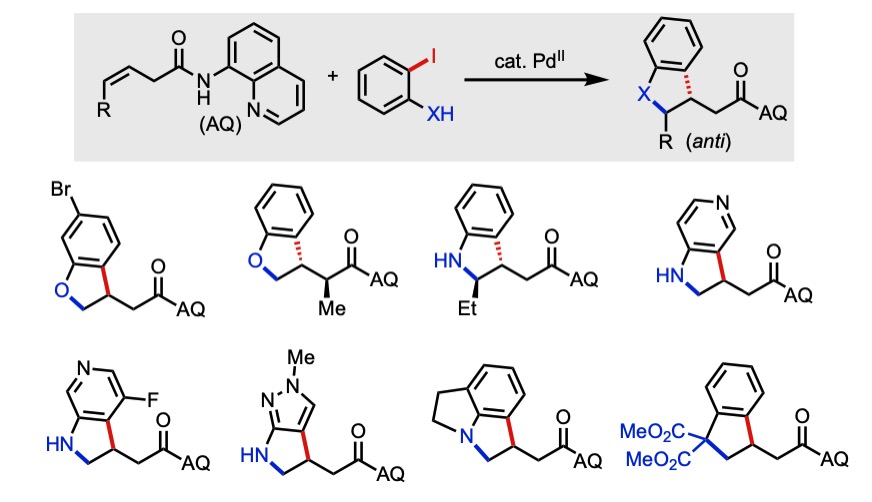
87. Apolinar, O.; Tran, V. T.; Kim, N.; Schmidt, M. A.; Derosa, J.; Engle, K. M. “Sulfonamide Directivity Enables Ni-Catalyzed 1,2-Diarylation of Diverse Alkenyl Amines,” ACS Catal. 2020, 10, 14234–14239.
• Pre-print uploaded to ChemRxiv on Jul. 10, 2020 (DOI: 10.26434/chemrxiv.12642803)
• Highlighted as an item of interest in OPR&D
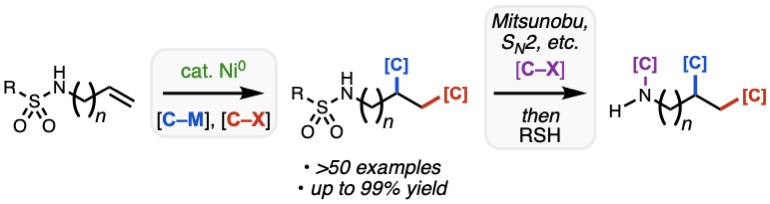
86. Kang, T.; Erbay, T. G.; Xu, K. L.; Gallego, G. M.; Burtea, A.; Nair, S. K.; Patman, R. L.; Zhou R.; Sutton, S. C.; McAlpine, I. J.; Liu, P.; Engle, K. M. “Multifaceted Substrate–Ligand Interactions Promote the Copper-Catalyzed Hydroboration of Benzylidenecyclobutanes and Related Compounds,” ACS Catal. 2020, 10, 13075–13083.
• Pre-print uploaded to ChemRxiv on Aug. 12, 2020 (DOI: 10.26434/chemrxiv.12788390)
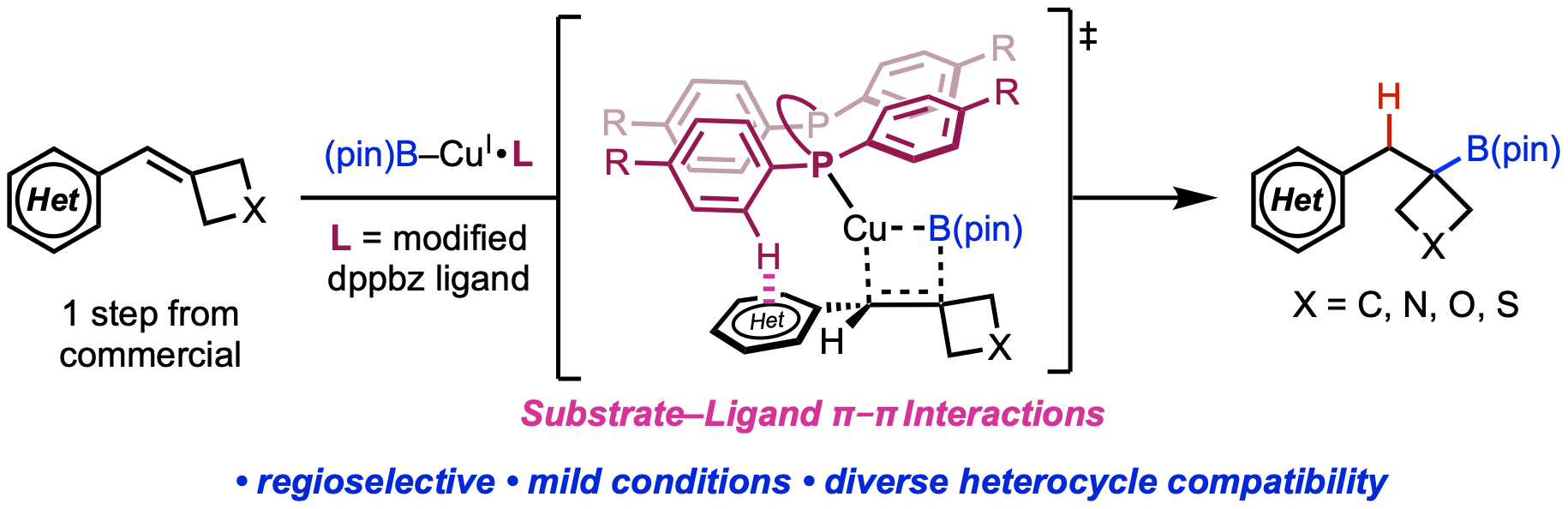
85. Wang, X.†; Li, Z.-Q.†; Mai, B. K.†; Gurak, J. A., Jr.; Xu, J. E.; Tran, V. T.; Ni, H.-Q.; Liu, Z.; Liu, Z.; Yang, K. S.; Xiang, R; Liu, P.; Engle, K. M. “Controlling Cyclization Pathways in Palladium(II)-Catalyzed Intramolecular Alkene Hydrofunctionalization via Substrate Directivity,” Chem. Sci. 2020, 11, 11307–11314. (†Authors contributed equally)
• Pre-print uploaded to ChemRxiv on Apr. 8, 2020 (DOI: 10.26434/chemrxiv.12090402)
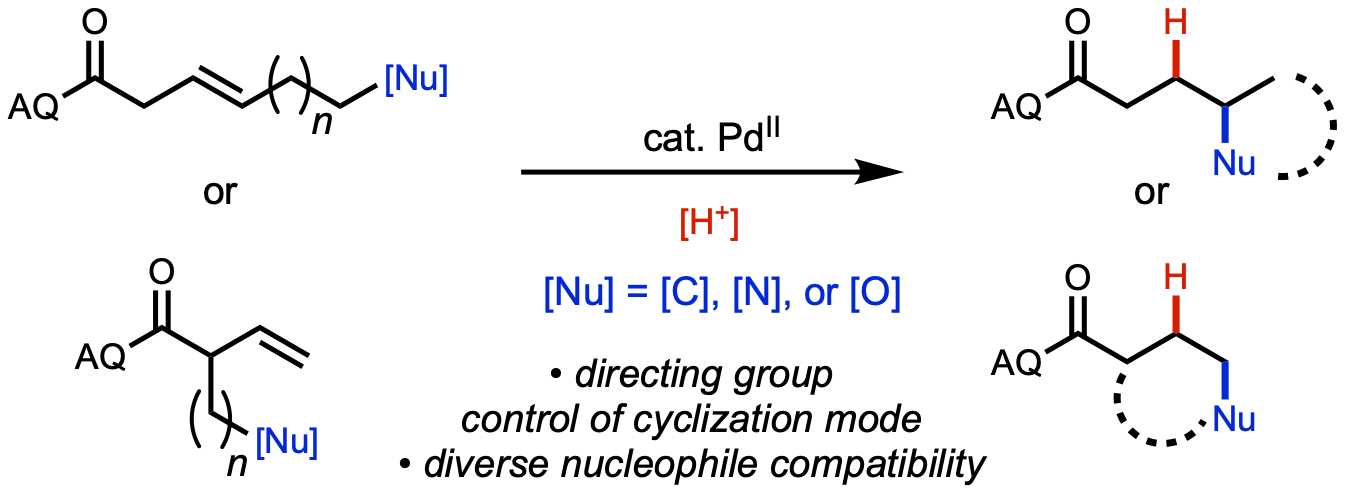
84. Li, Z.-Q.; Fu, Y.; Deng, R.; Tran, V. T.; Gao, Y.; Liu, P.; Engle, K. M. “Ligand-Controlled Regiodivergence in Nickel-Catalyzed Hydroarylation and Hydroalkenylation of Alkenyl Carboxylic Acids,” Angew. Chem. 2020, 132, 23506–23512; Angew. Chem. Int. Ed. 2020, 59, 23306–23312.
• Pre-print uploaded to ChemRxiv on Jul. 15, 2020 (DOI: 10.26434/chemrxiv.12650009)
• Highlighted in Synfacts

83. Gao, Y.; Wu, Z.-Q.; Engle, K. M. “Synthesis of Stereodefined 1,1-Diborylalkenes via Copper-Catalyzed Diboration of Terminal Alkynes,” Org. Lett. 2020, 22, 5235–5239.
• Pre-print uploaded to ChemRxiv on Apr. 15, 2020 (DOI: 10.26434/chemrxiv.12133404)
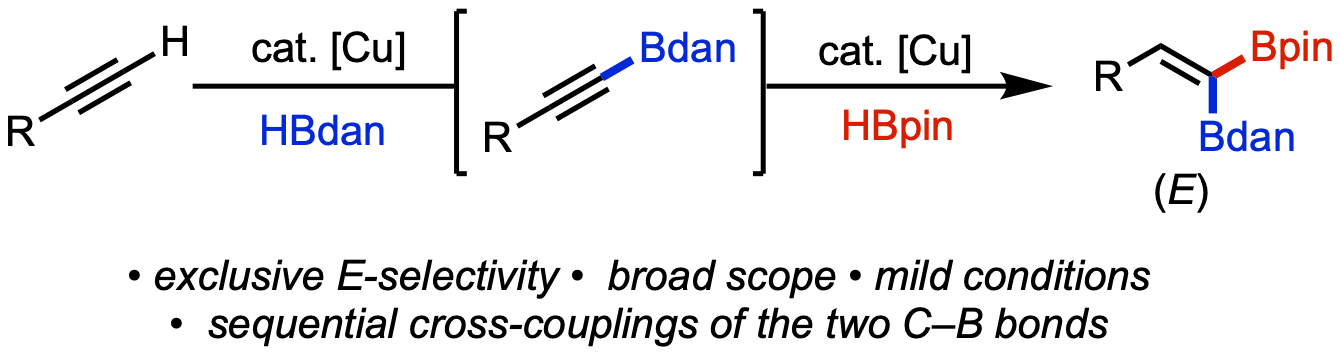
82. Vasquez, A. M.; Gurak, J. A., Jr.; Joe, C. L.; Cherney, E. C.; Engle, K. M. “Catalytic α-Hydroarylation of Acrylates and Acrylamides via an Interrupted Hydrodehalogenation Reaction,” J. Am. Chem. Soc. 2020, 142, 10477–10484.
• Pre-print uploaded to ChemRxiv on Mar. 18, 2020 (DOI: 10.26434/chemrxiv.12003444.v1)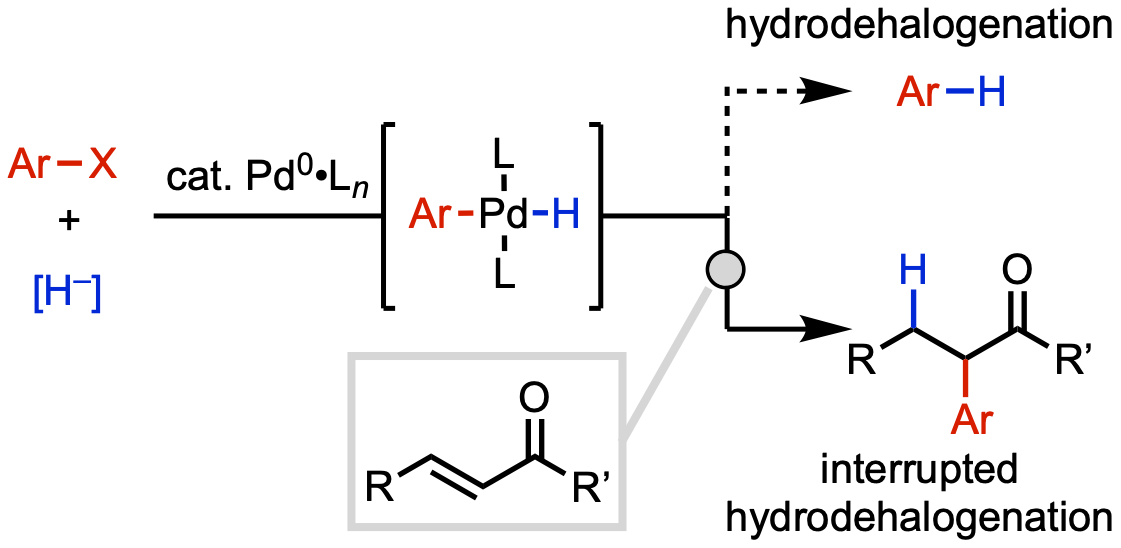
81. Oxtoby, L. J.; Li, Z.-Q.; Tran, V. T.; Erbay, T. G.; Deng, R.; Liu, P.; Engle, K. M. “A Transient Directing Group Strategy Enables Enantioselective Reductive Heck Hydroarylation of Alkenes,” Angew. Chem. 2020, 132, 8970–8975; Angew. Chem. Int. Ed. 2020, 59, 8885–8890.
• Pre-print uploaded to ChemRxiv on Sept. 11, 2019 (DOI: 10.26434/chemrxiv.9795338)
• Highlighted in Synfacts
• Highlighted by Chem-Station chemistry blog

80. Derosa, J.; Apolinar, O.; Kang, T.; Tran, V. T.; Engle, K. M. “Recent Developments in Nickel-Catalyzed Intermolecular Dicarbofunctionalization of Alkenes,” Chem. Sci. 2020, 11, 4287–4296. (Invited Review).
• Selected for the 2020 Hot Article Collection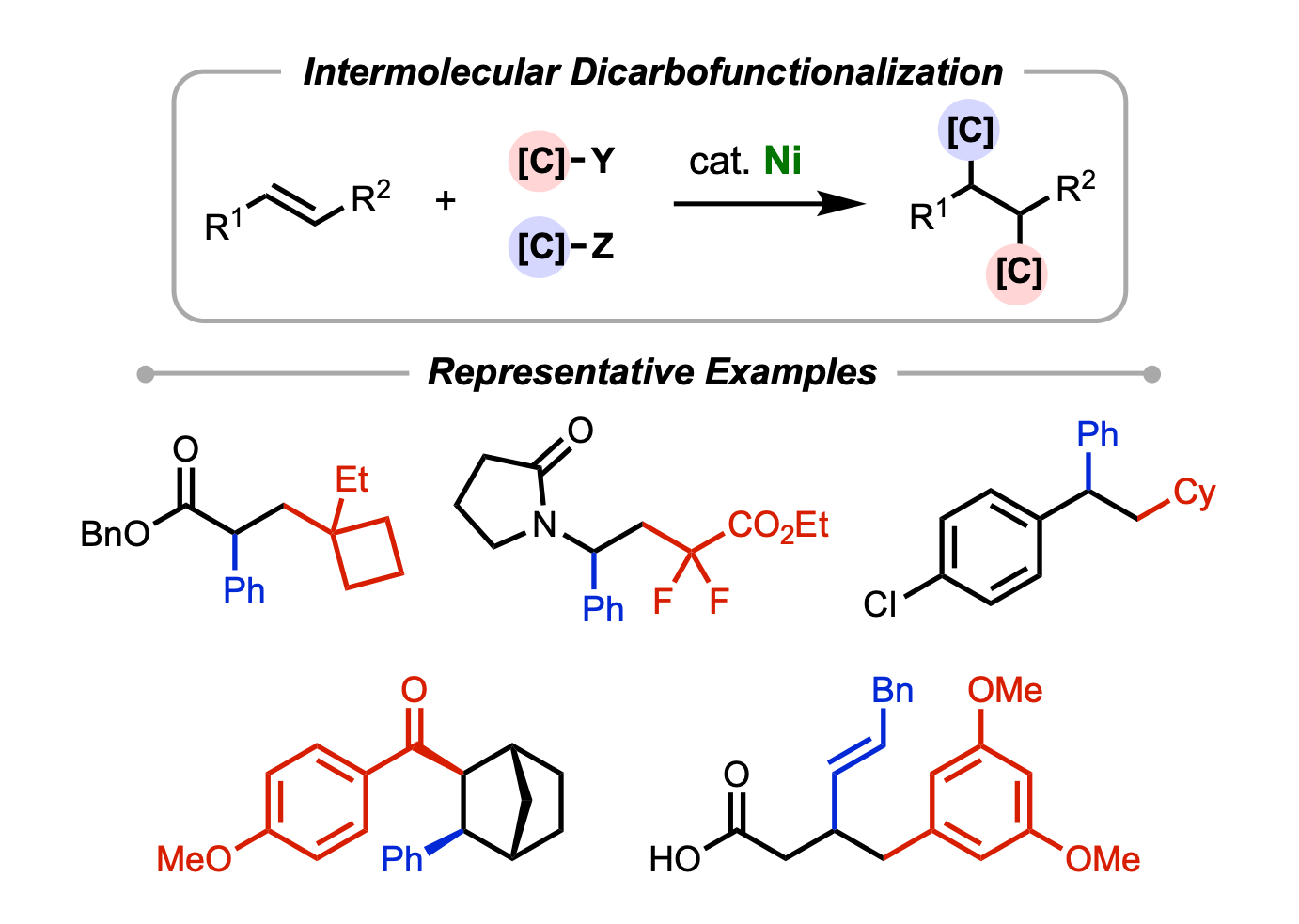
79. Liu, Z.; Gao, Y.; Zeng, T.; Engle, K. M.“Transition-Metal-Catalyzed 1,2-Carboboration of Alkenes: Strategies, Mechanisms, and Stereocontrol,” Isr. J. Chem. 2020, 60, 219–229.
• Special issue honoring 2019 Wolf Prize recipients, Profs. Stephen L. Buchwald and John F. Hartwig.

78. Tran, V. T.; Li, Z.-Q.; Apolinar, O.; Derosa, J.; Wisniewski, S. R.; Joannou, M. V.; Eastgate, M. D.; Engle, K. M. “Ni(COD)(DQ): An Air-Stable 18-Electron Ni(0)–Olefin Precatalyst,” Angew. Chem. 2020, 132, 7479–7483; Angew. Chem. Int. Ed. 2020, 59, 7409–7413.
• Selected as a Hot Paper
• Highlighted in Nat. Rev. Chem.
• Highlighted as an item of interest by OPR&D

77. Tran, V. T.; Li, Z.-Q.; Gallagher, T. J.; Derosa, J.; Liu, P.; Engle, K. M.“Integrating Allyl Electrophiles into Nickel-Catalyzed Conjunctive Cross-Coupling,” Angew. Chem. 2020, 132, 7095–7100; Angew. Chem. Int. Ed. 2020, 59, 7029–7034.
• Selected as a Hot Paper
• Pre-print uploaded to ChemRxiv on Aug. 25, 2019 (DOI: 10.26434/chemrxiv.9722348.v1)


75. Gao, D.-W.†; Gao, Y.†; Shao, H.; Qiao, T.-Z.; Wang, X.; Sanchez, B.; Chen, J. S.; Liu, P.; Engle, K. M.“Cascade CuH-Catalyzed Conversion of Alkynes to Enantioenriched 1,1-Disubstituted Products,” Nat. Catal. 2020, 3, 23–29.(†Authors contributed equally).
• Highlighted on CBG Blog
• Preprint uploaded to ChemRxiv on Apr. 9, 2019 (DOI: 10.26434/chemrxiv.7961633)
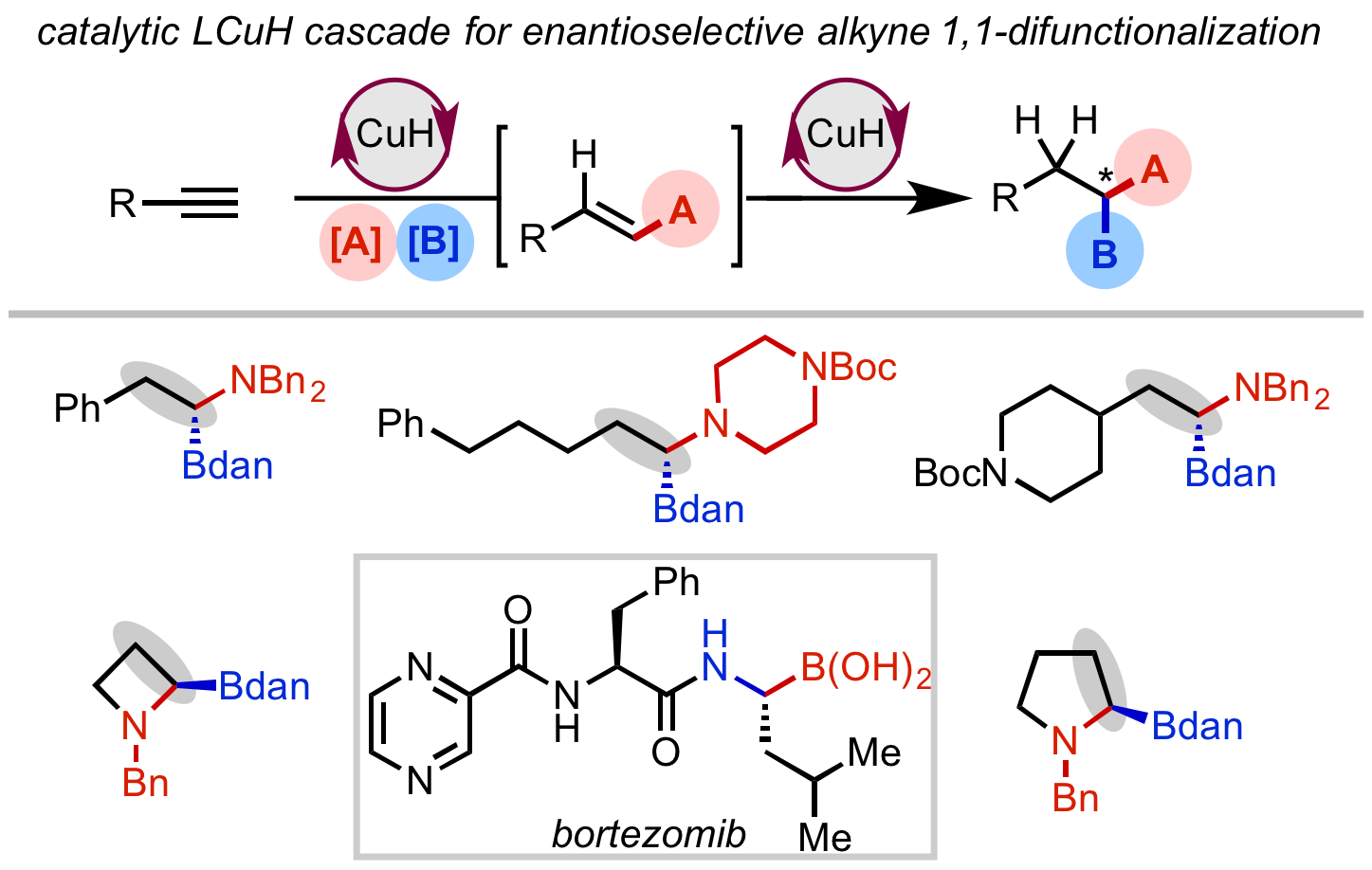
74. Derosa, J.†; Kang, T.†; Tran, V. T.; Wisniewski, S. R.; Karunananda, M. K.; Jankins, T. C.; Xu, K. L; Engle, K. M. “Nickel-Catalyzed 1,2-Diarylation of Alkenyl Carboxylates A Gateway to 1,2,3-Trifunctionalized Building Blocks,” Angew. Chem. 2020, 132, 1217–1221; Angew. Chem. Int. Ed. 2020, 59, 1201–1205. (†Authors contributed equally)
• Preprint uploaded to ChemRxiv of Sept. 25, 2019 (DOI: 10.26434/chemrxiv.9900980)

73. Lv, H.; Kang, H.; Zhou, B.; Xue, X.; Engle, K. M.; Zhao, D. “Nickel(0)-Catalyzed Intermolecular Oxidative Heck Arylation Driven by Transfer Hydrogenation,” Nat. Commun. 2019, 10, 5025.
• Highlighted in Synfacts

72. Medina, J. M.†; Kang, T.†; Erbay, T. G.; Shao, H.; Gallego, G. M.; Yang, S.; Tran-Dubé, M.; Richardson, P. F.; Derosa, J.; Helsel, R.; Patman, R. L.; Wang, F.; Ashcroft, C. P.; Braganza, J. F.; McAlpine, I.; Liu, P.; Engle, K. M.; “Cu-Catalyzed Hydroboration of Benzylidenecyclopropanes: Reaction Optimization, (Hetero)Aryl Scope, and Origins of Pathway Selectivity,” ACS Catal. 2019, 9, 11130–11136. (†Authors contributed equally)
• Preprint uploaded to ChemRxiv on June 27, 2019 (DOI: 10.26434/chemrxiv.8341016)
71. Liu, Z.; Chen, J.; Lu, H.-X.; Li, X.; Gao, Y.; Coombs, J. R.; Goldfogel, M.; Engle, K. M.“Pd(0)-Catalyzed Directed syn-1,2-Carboboration and -Silylation: Alkene Scope, Applications in Dearomatization, and Stereocontrol via a Chiral Auxiliary,” Angew. Chem. 2019, 131, 17224–17229; Angew. Chem. Int. Ed. 2019, 58, 17068–17073.
• Pre-print uploaded to ChemRxiv on Jul. 19, 2019 (DOI: 10.26434/chemrxiv.8865143)
• Highlighted in Synfacts

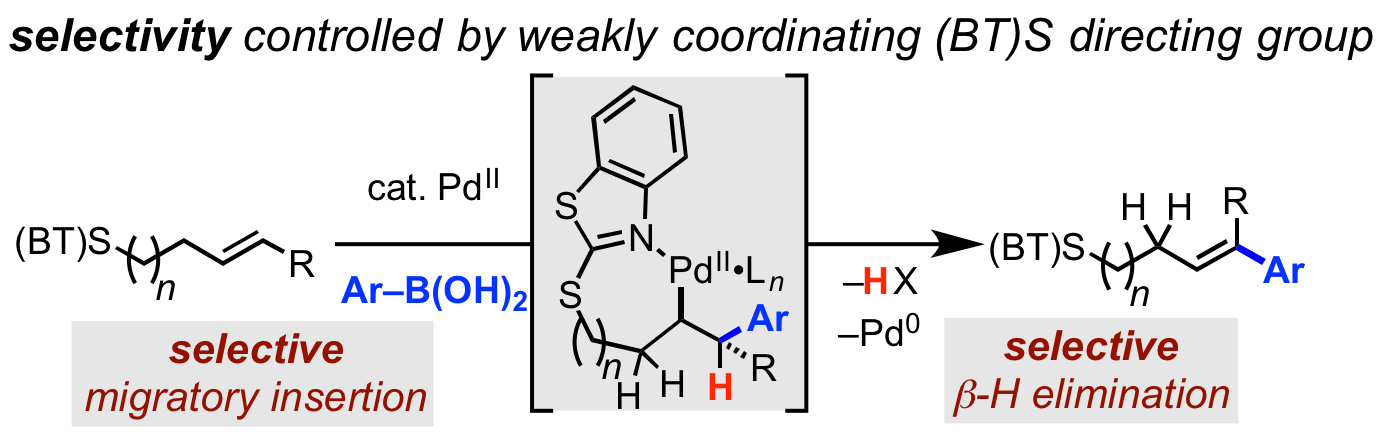
70. Romine, A. M.; Yang, K. S.; Karunananda, M. K.; Chen, J. S.; Engle, K. M. “Synthetic and Mechanistic Studies of the a Versatile Heteroaryl Thioether Directing Group for Pd(II) Catalysis,” ACS Catal. 2019, 9, 7626–7640.
• Preprint uploaded to ChemRxiv on Mar. 29, 2019. (DOI: 10.26434/chemrxiv.7927166)
• Highlighted in Synfacts

68. Jankins, T. C.†; Qin, Z.-Y.†; Engle, K. M.; “A Practical Method for N-Alkylation of Phosphinic (Thio)amides with Alcohols via Transfer Hydrogenation,” Tetrahedron 2019, 75, 3272–3281. (†Authors contributed equally).
• Special Issue for Ryan Shenvi’s 2019 Tetrahedron Young Investigator Award

67. Liu, Z.; Li, X.; Zeng, T.; Engle, K. M. “Directed, Palladium(II)-Catalyzed Enantioselective anti-Carboboration of Alkenyl Carbonyl Compounds,” ACS Catal. 2019, 9, 3260–3265.
• Preprint uploaded to ChemRxiv on Jan. 11, 2019. (DOI: 10.26434/chemrxiv.7577597)
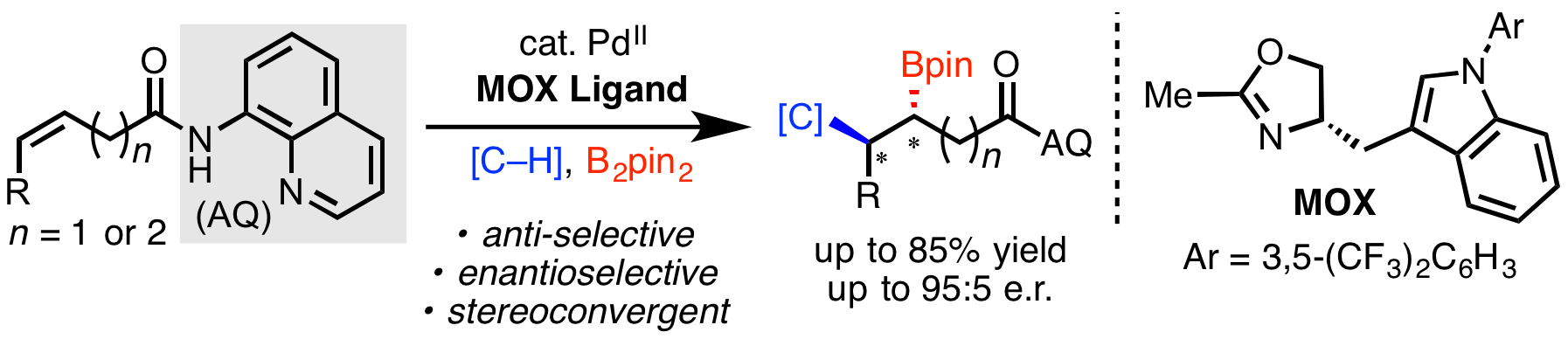
66. Nimmagadda, S. K.†; Liu, M.†; Karunananda, M. K.†; Gao, D.-W.; Apolinar, O.; Chen, J. S.; Liu, P.; Engle, K. M. “Catalytic, Enantioselective α-Alkylation of Azlactones with Non-Conjugated Alkenes via Directed Nucleopalladation,” Angew. Chem. 2019, 131, 3963–3967; Angew. Chem. Int. Ed. 2019, 58, 3923–3927; (†Authors contributed equally).
• Selected as a Hot Paper
• Highlighted by CSUSM NewsCenter
• Preprint uploaded to ChemRxiv on Nov. 6, 2018 (DOI: 10.26434/chemrxiv.7313615)
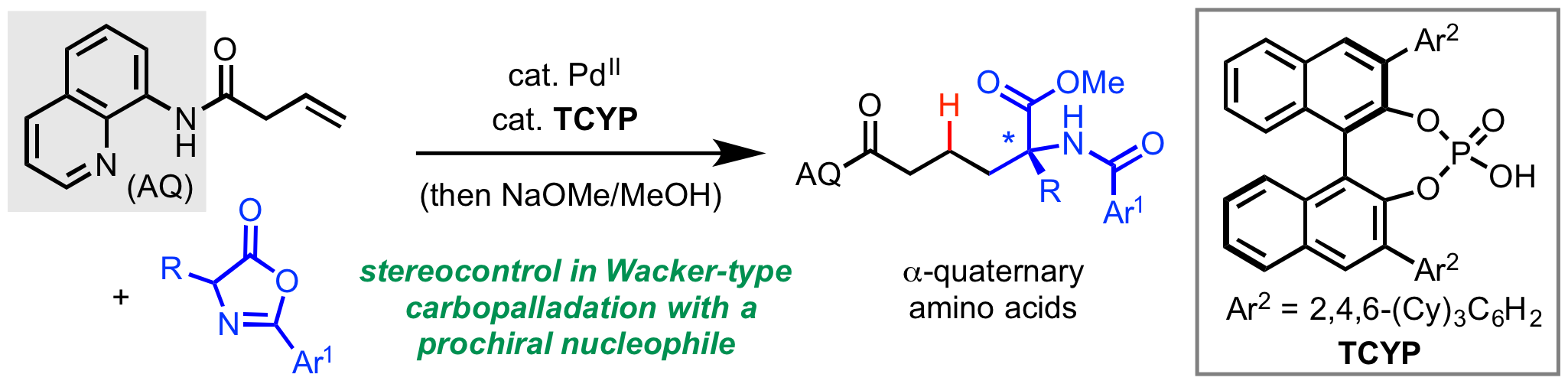
65. van der Puyl, V. A.; Derosa, J.; Engle, K. M. “Directed, Nickel-Catalyzed Umpolung 1,2-Carboamination of Alkenyl Carbonyl Compounds,” ACS Catal. 2019, 9, 224–229.
• Preprint uploaded to ChemRxiv on Nov. 5, 2018 (DOI: 10.26434/chemrxiv.7301453)

64. Derosa, J.; Kleinmans, R.†; Tran, V. T.†; Karunananda, M. K.; Wisniewski, S. R.; Eastgate, M. D.; Engle, K. M. “Nickel-Catalyzed 1,2-Diarylation of Simple Alkenyl Amides,” J. Am. Chem. Soc. 2018, 140, 17878–17883. (†Authors contributed equally).
• Preprint uploaded to ChemRxiv on Nov. 6, 2018 (DOI: 10.26434/chemrxiv.7300058)
• Highlighted by Chem-Station chemistry blog
• Featured in Organic Chemistry Portal

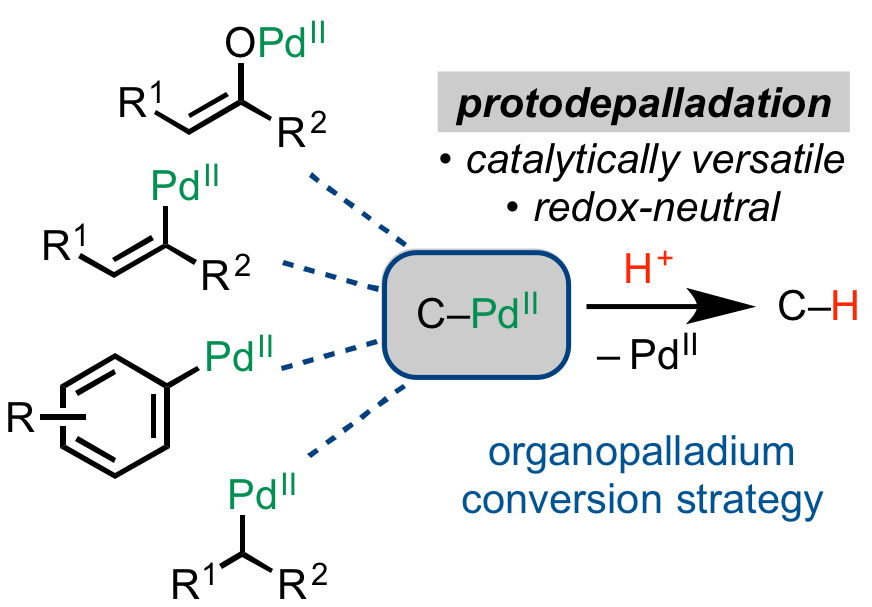
62. Matsuura, R.; Jankins, T. C.; Hill, D. E.; Yang, K. S.; Gallego, G. M.; Yang. S.; He, M.; Wang, F.; Marsters, R.; McAlpine, I.; Engle, K. M. “Palladium(II)-Catalyzed γ-Selective Hydroarylation of Alkenyl Carbonyl Compounds with Arylboronic Acids,” Chem. Sci. 2018, 9, 8363–8368.
• Selected for the 2018 Chemical Science HOT Article Collection
• Preprint uploaded to ChemRxiv on Apr. 16, 2018 (DOI: 10.26434/chemrxiv.5885203)
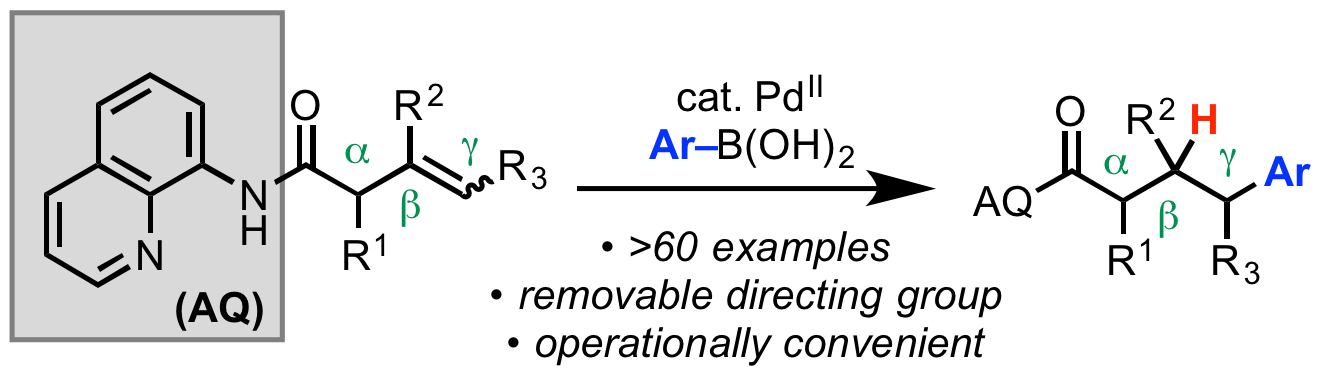
61. Gurak, J. A., Jr.; Engle, K. M. “Practical Intermolecular Hydroarylation of Diverse Alkenes via Reductive Heck Coupling,” ACS Catal. 2018, 8, 8987–8992.
• Preprint uploaded to ChemRxiv on Jun. 20, 2018 (DOI:10.26434/chemrxiv.6619223)

60. Tran, V. T.; Gurak, J. A., Jr.; Yang, K. S.; Engle, K. M. “Activation of Diverse Carbon–Heteroatom and Carbon–Carbon Bonds via Palladium(II)-Catalyzed β-X Elimination,” Nat. Chem. 2018, 10, 1126–1133.
• Nature Chemistry Community “Behind the Paper” Highlight by Van
• Highlighted by X-mol chemistry blog
• Highlighted by Synfacts
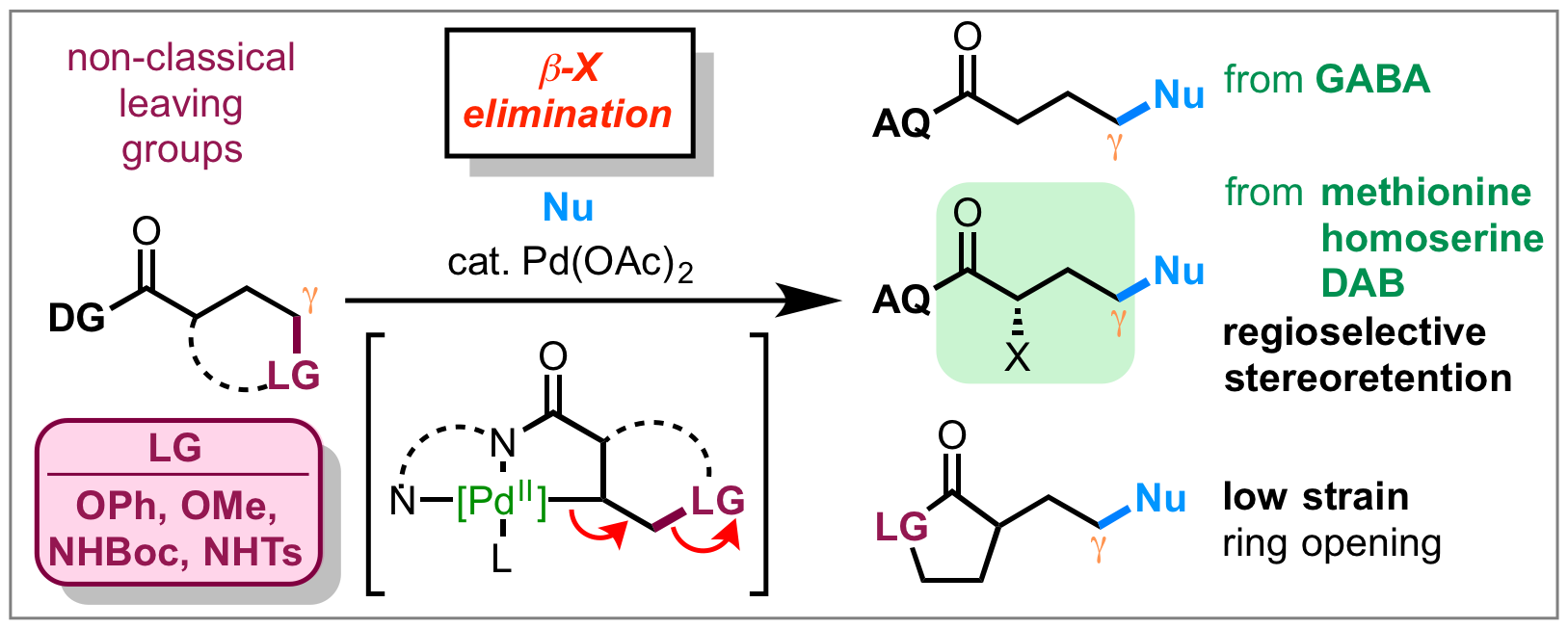
59. Gao, D.-W.; Vinogradova, E. V.; Nimmagadda, S. K. †; Medina, J. M.†; Xiao, Y.; Suciu, R. M.; Cravatt, B. F.; Engle, K. M. “Direct Access to Versatile Electrophiles via Catalytic Oxidative Cyanation of Alkenes,” J. Am. Chem. Soc. 2018, 140, 8069–8073. (†Authors contributed equally).
• Highlighted by X-mol chemistry blog
• Featured in Organic Chemistry Portal and Organic Chemistry Highlights (September 2019)



56. Derosa, J.; van der Puyl; V. A.; Tran, V. T.; Liu, M.; Engle, K. M. “Directed Nickel-Catalyzed 1,2-Dialkylation of Alkenyl Carbonyl Compounds,” Chem. Sci. 2018, 9, 5278–5283.
• Preprint uploaded to ChemRxiv on Jan. 23, 2018 (DOI: 10.26434/chemrxiv.5807427.v2.)
• Highlighted in Synfacts.
• Featured in ChemRxiv Themed Collection.
• Featured in the 2018–2019 Most Popular Organic Chemistry Articles Collection
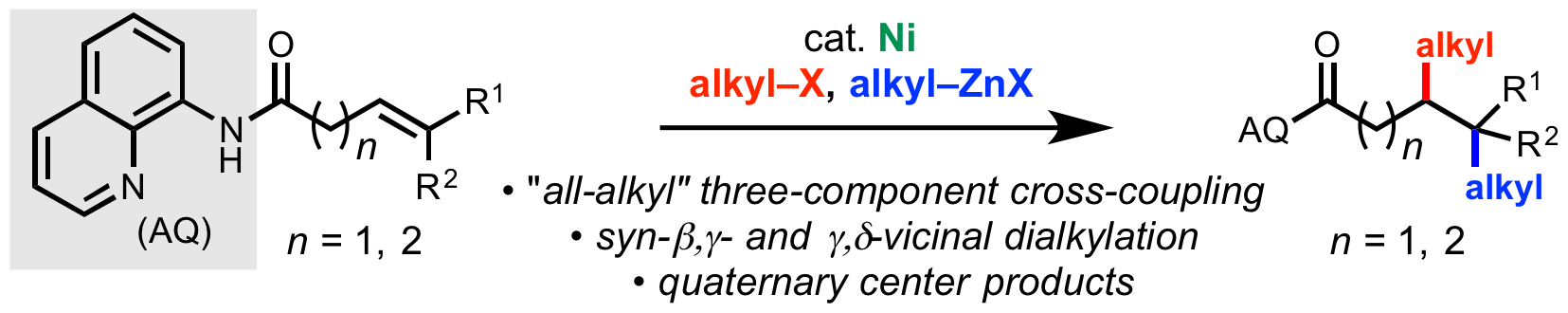

54. Gao, D.-W.; Xiao, Y.; Liu, M.; Liu, Z.; Karunananda, M. K.; Chen, J. S; Engle, K. M. “Catalytic, Enantioselective Synthesis of Allenyl Boronates,” ACS Catal. 2018, 8, 3650–3654.
• Preprint uploaded to ChemRxiv on Feb. 14, 2018 (DOI: 10.26434/chemrxiv.5885203.v1).
• Highlighted in the Jan/Feb ChemRxiv Round-up.
• Highlighted in Synfacts.
• Featured in Organic Chemistry Highlights (October 2018).

53. Derosa, J.†; O’Duill, M. L.†; Holcomb, M.; Boulous, M. N.; Patman, R. L.; Wang, F.; Tran-Dubé, M.; McAlpine, I.; Engle, K. M. “Copper-Catalyzed Chan–Lam Cyclopropylation of Phenols and Aza-Heterocycles,” J. Org. Chem. 2018, 83, 3417–3425. (†Authors contributed equally).
• Selected as a Featured Article.
• Highlighted in Synfacts.
52. Liu, Z.; Ni, H.-Q.; Zeng, T.; Engle, K. M. “Catalytic Carbo- and Aminoboration of Alkenyl Carbonyl Compounds via 5- and 6-Membered Palladacycles,” J. Am. Chem. Soc. 2018, 140, 3223–3227.
• Highlighted by X-Mol chemistry blog.
51. O’Duill, M. L.; Matsuura, R.; Wang, Y.; Turnbull, J. L.; Gurak, J. A., Jr.; Gao, D.-W.; Lu, G.; Liu, P.; Engle, K. M. “Tridentate Directing Groups Stabilize 6-Membered Palladacycles in Catalytic Alkene Hydrofunctionalization,” J. Am. Chem. Soc. 2017, 139, 15576–15579.
• Highlighted by X-mol chemistry blog.
49. Liu, Z.; Wang, Y.; Wang, Z.; Zeng, T.; Liu, P.; Engle, K. M. “Catalytic Intermolecular Carboamination of Unactivated Alkenes via Directed Aminopalladation,” J. Am. Chem. Soc. 2017, 139, 11261–11270.
• Highlighted as an item of interest by OPR&D.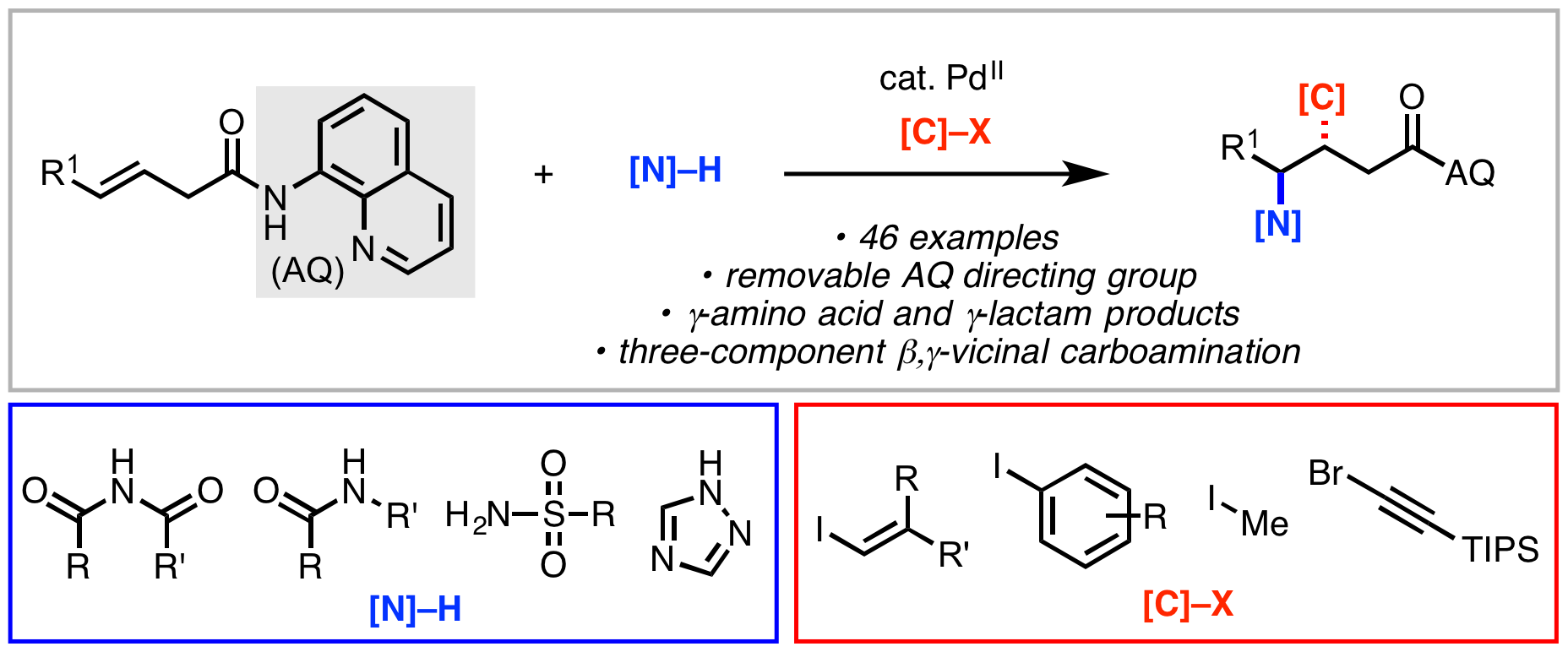
48. Derosa, J.†; Tran, V. T.†; Boulous, M. N.; Chen, J. S.; Engle, K. M. “Nickel-Catalyzed β,γ-Dicarbofunctionalization of Alkenyl Carbonyl Compounds via Conjunctive Cross-Coupling,” J. Am. Chem. Soc. 2017, 139, 10657–10660. (†Authors contributed equally).
• Highlighted in ChemistryViews magazine.
• Highlighted in Synfacts.
• Highlighted as an item of interest by OPR&D.
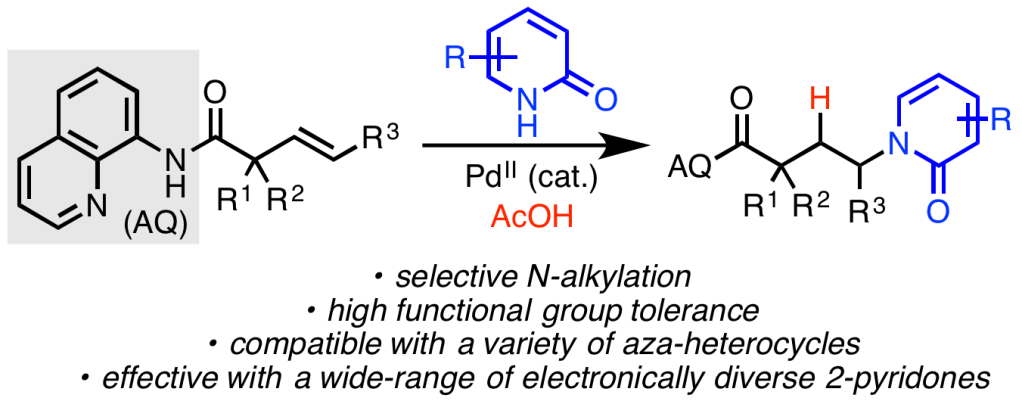
47. Gurak, J. A., Jr.; Tran, V. T.; Sroda, M. M.; Engle, K. M. “N-Alkylation of 2-Pyridone Derivatives via Palladium(II)-Catalyzed Directed Alkene Hydroamination,” Tetrahedron 2017, 73, 3636–3642.
• Special issue for Ang Li’s 2017 Tetrahedron Young Investigator Award
46. Derosa, J.; Cantu, A. L.; Boulous, M. N.; O’Duill, M. L.; Turnbull, J. L.; Liu, Z.; De La Torre, D. M.; Engle, K. M. “Directed Palladium(II)-Catalyzed anti-Hydrochlorination of Unactivated Alkynes with HCl,” J. Am. Chem. Soc. 2017, 139, 5183–5193.
45. Liu, Z.; Zeng, T.; Yang, K. S.; Engle, K. M. “β,γ-Vicinal Dicarbofunctionalization of Alkenyl Carbonyl Compounds via Directed Nucleopalladation,” J. Am. Chem. Soc. 2016, 138, 15122–15125.
• Featured in Organic Chemistry Highlights (September 2017)
• Highlighted as an item of interest by OPR&D.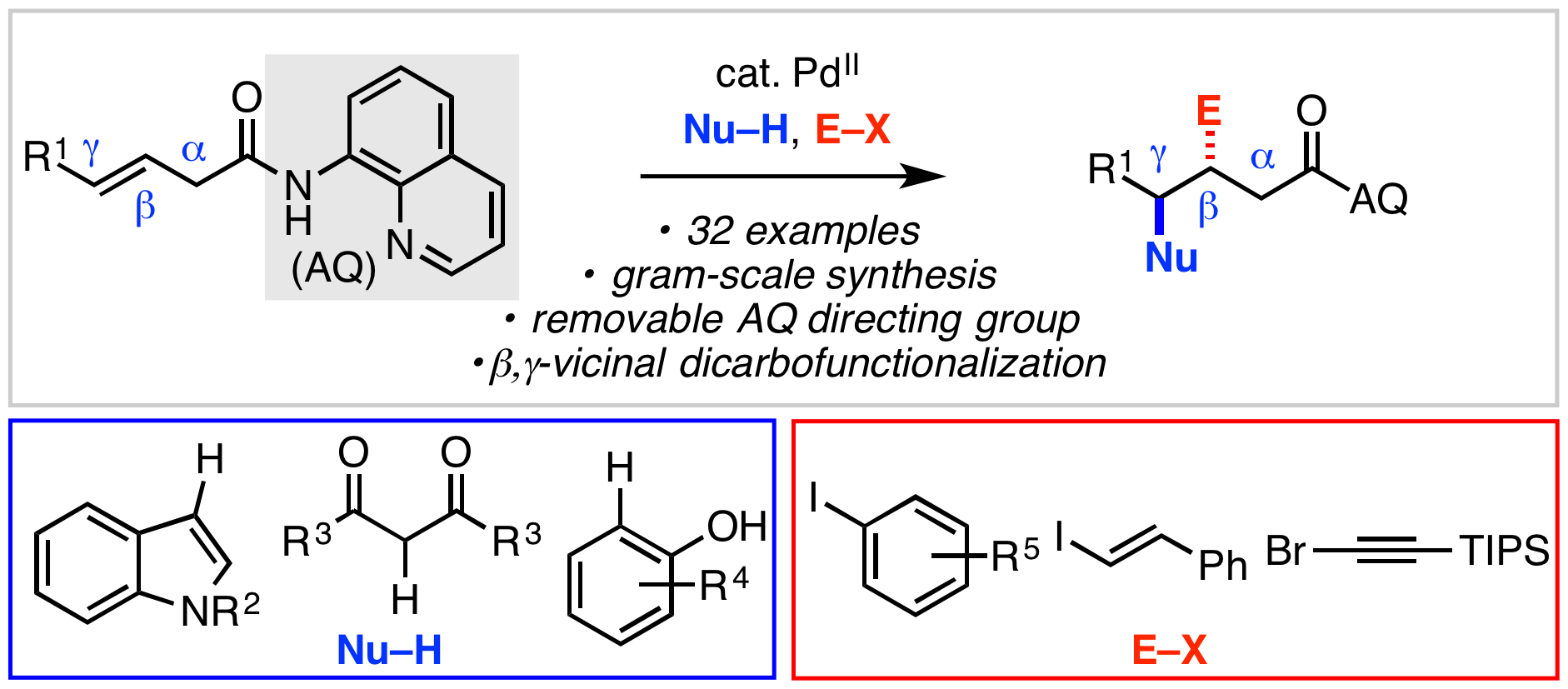
43. Liu, Z.; Derosa, J.; Engle, K. M. “Palladium(II)-Catalyzed Regioselective syn-Hydroarylation of Disubstituted Alkynes Using a Removable Directing Group,” J. Am. Chem. Soc. 2016, 138, 13076–13081.
• Highlighted in Synfacts.
UNDERGRADUATE, GRADUATE, AND POSTDOCTORAL
30. Engle, K. M.; Dastbaravardeh, N.; Thuy-Boun, P. S.; Wang, D.-H.; Sather, A. C.; Yu, J.-Q. “Ligand-Accelerated ortho-C–H Olefination of Phenylacetic Acids,” Org. Synth. 2015, 92, 58–75.
BOOKS AND BOOK CHAPTERS
OTHER WRITING
Engle, K. M. “The Science of Writing,” LSA Young Alumni Blog, 25 May 2016.





