This week the editorial board of the RSC journal Chem. Soc. Rev. announced the joint winners of the 2025 ChemSocRev Pioneering Investigator lectureship, recognizing Keary together with Prof. Garrett Miyake (Colorado State). Congrats to both awardees!
Stable and Soluble bis(perfluorophenyl)nickel(II) precatalysts Described in Newest pre-print
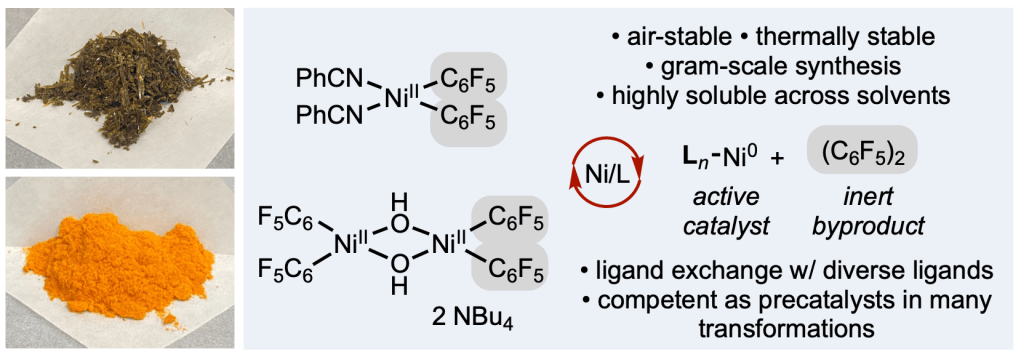
Over the past five years, our efforts to develop robust nickel precatalysts have revealed a key insight: stability alone isn’t enough. For practical, broadly useful catalysis, solubility across a wide range of organic solvents is just as critical.
In our latest preprint, we introduce a new family of bis(perfluoroaryl)nickel(II) precatalysts that combine exceptional stability, high solubility, and operational simplicity. Inspired by classic inorganic chemistry strategies, this design cleanly releases an inert (C₆F₅)₂ byproduct upon catalyst initiation—streamlining activation while maintaining performance.
The project was led by former postdoctoral scholar Nana Kim (now at Bristol Myers Squibb) and graduate student Aimee Bangerter, and brought together a talented, multidisciplinary team spanning Scripps Research, University of California San Diego, and Bristol Myers Squibb.
Congratulations to everyone involved in this outstanding collaborative effort!
🧪 ChemRxiv pre-print: https://chemrxiv.org/doi/full/10.26434/chemrxiv.15000413/v1
Welcome to our newest undergrad, Yuan Xu
The Engle Lab is delighted to welcome Yuan Xu, a visiting undergraduate from Zhejiang University. At his home institution, Yuan conducts research in the laboratory of Professor Bing-Feng Shi.
In our lab, Yuan will be working under the mentorship of Wen-Ji on nickel pre-catalyst development and nickel-catalyzed olefin difunctionalization. We’re excited to have him join us and look forward to the contributions he’ll make during his time here.
Welcome to the Engle Lab, Yuan!
Masa finishes his postdoc
We’re excited to celebrate Masa as he wraps up his postdoctoral fellowship in the Engle Lab this week!
During his year with us, Masa developed innovative strategies for nickel-catalyzed 1,2-diarylation, advancing the lab’s work in powerful and practical ways. Beyond the bench, he generously shared insights from his experience as a medicinal chemist, enriching our group with real-world perspectives on drug discovery and development.
Masa now returns to Shionogi to continue his career in drug discovery, where he’ll carry forward his expertise in nickel catalysis and the collaborations built here.
Thank you, Masa, for your scientific contributions, mentorship, and the many great memories. We wish you every success in this next chapter!


Flexible directing group study published in JACS
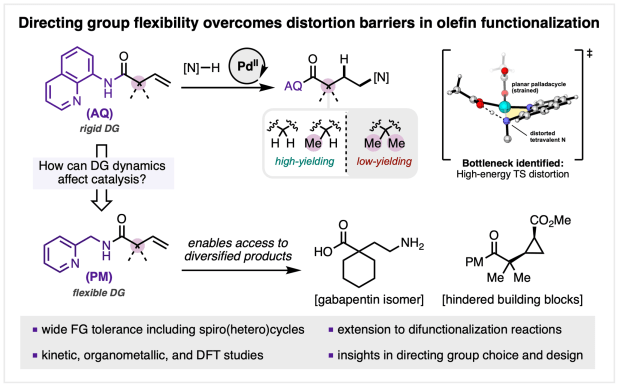
We’re excited to announce that our collaborative study “Mechanistically Guided Functionalization of α,α-Disubstituted Alkenyl Amides Enabled by a Conformationally Flexible Directing Auxiliary” has been published in final form in J. Am. Chem. Soc.
In this work, Al, Gift, Shijia, and team demonstrate how a conformationally flexible directing auxiliary can overcome long-standing reactivity barriers in PdII-catalyzed alkene functionalization. By marrying detailed mechanistic analysis with method development, this strategy enables efficient and regioselective hydroamination of challenging α,α-disubstituted alkenyl amides, broadening the scope of substrates and catalytic transformations accessible with directing group assistance.
Congratulations to all the authors involved from Scripps Research, the University of Pittsburgh, Bristol Myers Squibb, and Enamine! This publication reflects our ongoing commitment to uncovering mechanistic principles that drive innovative synthetic methods.
📄 Read the paper in Journal of the American Chemical Society: https://pubs.acs.org/doi/10.1021/jacs.5c21886
🧪 Original preprint on ChemRxiv (December 2025): https://chemrxiv.org/doi/10.26434/chemrxiv-2025-bsn9s
Nickel-Catalyzed 1,2-Alkylarylation for Encoded Polymer Synthesis – Paper now in press in ACS Central Science
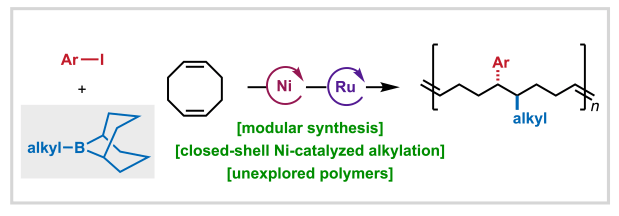
We’re pleased to share that Anne, Aimee, Shijia, and Ethan’s study developing a nickel-catalyzed 1,2-alkylarylation method for accessing sequence encoded monomers has been published in ACS Central Science!
In this report, we describe how B-alkyl-borabicyclo[3.3.1]nonane (Alkyl-9-BBN) reagents are uniquely effective for alkyl group transfer in closed-shell Ni(0)/Ni(II) cycles, enabling selective functionalization of cyclic dienes without competitive ring closure. This work exemplifies how innovative reaction design combined with mechanistic understanding can expand the frontiers of selective chemical synthesis and catalysis.
Congratulations to all co-authors and collaborators from Scripps Research, University of Pittsburgh, Georgia Tech, and Bristol Myers Squibb for this accomplishment! This publication continues to underscore our lab’s dedication to developing catalytic methods with broad utility in organic synthesis and materials chemistry.
📄 Read the paper in ACS Central Science: https://pubs.acs.org/doi/10.1021/acscentsci.5c02173
🧪 Original preprint on ChemRxiv (October 2025): https://chemrxiv.org/engage/chemrxiv/article-details/68e901addfd0d042d1dace4c
Sourav completes his postdoc
Congratulations to Sourav on completing his two-year appointment as a CNRS-supported postdoctoral fellow, jointly mentored with Prof. Rodolphe Jazzar at SDSU!
During his time in the lab, Sourav developed new carbene-ligated nickel and copper catalysts and discovered innovative methods for π-bond functionalization. His creativity, dedication, and collaborative spirit made a lasting impact on both our research and our group culture.
We’re excited that Sourav is beginning the next chapter of his career as an industrial chemist at Sai Life Sciences. Thank you, Sourav, for your outstanding contributions—we wish you continued success in all that’s ahead!


Collaborative work published in Nature Chemical Biology
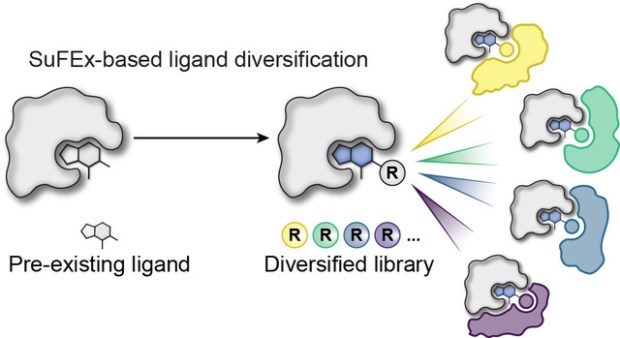
We’re proud to share that our contribution to the collaborative study led by Prof. Michael Erb’s lab entitled, “High-throughput ligand diversification to discover chemical inducers of proximity” has been published in Nature Chemical Biology.
In this work, we joined forces with an outstanding interdisciplinary team to implement a high-throughput small-molecule ligand diversification strategy, expanding thousands of analogs of known binders to systematically explore and discover chemical inducers of proximity (CIPs) such as molecular glues. By coupling sulfur(VI) fluoride exchange (SuFEx) chemistry with phenotypic degradation screens, we identified new compounds that selectively recruit the CRL4CRBN E3 ubiquitin ligase to previously untargeted protein partners—opening new avenues in proximity pharmacology. Our lab’s contribution centered on troubleshooting a challenging C–N coupling using Pd(COD)(DQ).
Huge congratulations to all authors and collaborators! This publication highlights an exciting platform for discovering next-generation chemical modulators of biology. Stay tuned as additional work from this and other chemical biology collaborations from the lab.
🧪 Original preprint on BioRxiv (September 2024): https://www.biorxiv.org/content/10.1101/2024.09.30.615685v1
📄 Read the paper in Nature Chemical Biology: https://www.nature.com/articles/s41589-025-02137-2
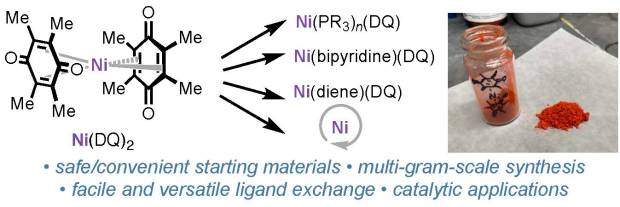
Bis(duroquinone)nickel paper in press in Organometallics
We’re excited to announce that the manuscript previously shared as a ChemRxiv pre-print—“Ni(DQ)₂: A Useful Gateway to Zero-Valent Nickel Complexes”—has now been accepted for publication in Organometallics.
In this work, we teamed up with collaborators at Bristol Myers Squibb, UCSD, and Cornell to develop a practical and scalable approach to synthesize Ni(DQ)₂ (DQ = duroquinone) from readily available nickel precursors, guided by DFT calculations. This method provides an accessible entry to zero-valent nickel complexes, which are valuable intermediates in organometallic synthesis and catalysis.
Huge congratulations to all authors and collaborators! This publication builds on the exciting progress our group has been making in air-stable nickel pre-catalysts.
Stay tuned for more updates as additional manuscripts from our group move through peer review!
📄 Read the paper in Organometallics: https://pubs.acs.org/doi/abs/10.1021/acs.organomet.5c00450
🧪 Original preprint on ChemRxiv (November 2025): https://chemrxiv.org/doi/10.26434/chemrxiv-2025-z69d2
Yuhang joins the lab as an undergraduate intern
The Engle Lab is excited to welcome our newest undergraduate researcher, Yuhang Zheng, a junior biochemistry major at University of California, San Diego.
Under the mentorship of Yiyao and Madison, Yuhang is eager to gain his first hands-on experience in organic chemistry research and synthetic methods development. We’re thrilled to have him join the team and look forward to seeing all he accomplishes.
Welcome to the lab, Yuhang!