Our new postdoc Yang Gao joins us from the Goldman Lab in Rutgers. We’re excited to have another great organometallic chemist join the group and teach us more about Iridium chemistry!
Month: April 2018
New Undergrad joins the Lab
New undergrad Ryan joins us from UCSD for his first position in a research lab. Ryan has already started working in the hood and is running columns under mentorship of Jose. Welcome to the lab Ryan!
Gamma-Selective Alkene Hydroarylation with Arylboronic Acids
As part of an ongoing collaboration with Pfizer, today we report a new method for achieving γ-selective hydroarylation of alkenyl carbonyl compounds, which is available as a pre-print on ChemRxiv (click here). This reaction is notable for its simplicity, practicality, and functional group tolerance. Because of the ubiquity of arylboronic acids in pharma and in academia, this protocol offers a powerful approach to appending an aryl group of one’s choosing to the γ-position of a carboxylic acid substrate. Congrats to all of the authors: Rei, Tanner, Kin, and Rohan from TSRI; and Gary, Shouliang, Mingying, Fen, and Indra from Pfizer. It was great working with the Pfizer team to field-test the method and to identify valuable heterarylboronic acids that would be of interest to medicinal chemists. Special shoutout to high school student co-author, Rohan, who worked with us earlier this year as part of his junior year internship. Great work, team! Stay tuned for the peer-reviewed version.

San Marcos Bridges Program Lab Visit
Undergraduate students from the San Marcos Bridges Program visited the Engle Lab to get glimpse into the typical work for PhD students in the sciences. Students listened to a lecture by Keary, then participated in a lab tour andtried setting up some air free chemistry in the glovebox. Thanks to the participating lab members for organizing the visit and showing the students the lab!

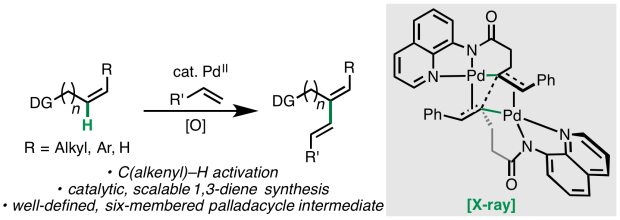
Catalytic 1,3-Diene Synthesis via C(alkenyl)–H Activation
In an Article appearing online today in J. Am. Chem. Soc., the Engle lab and Liu lab (U Pittsburgh) report a detailed study of palladium(II)-catalyzed C(alkenyl)–H activation, including optimization of a method to prepare structurally diverse 1,3-dienes, organometallic synthesis of key intermediates relevant to catalysis, and experimental/computational studies to interrogate the reaction mechanism. Congrats to the whole team: Mingyu, Pusu, and Malkanthi from Scripps; and Yanyan from Pitt. Special shout out to undergraduate co-author Pusu, who was a visiting student from Nankai university for six months. Thanks to the Liu lab for another fruitful collaboration! Way to go, everyone! Click here for a link to the paper.
Andrew wins 2018 NSF Graduate Research Fellowship
Congratulations to second-year graduate student Andrew Romine for being awarded an 2018 NSF Graduate Research Fellowship. Well done and well deserved!
For a complete list of awardees, click here.









