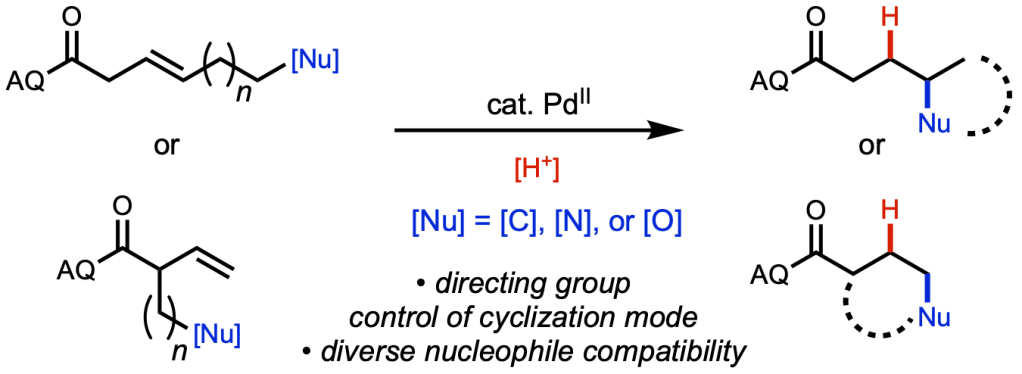
Xin, Zi-Qi, and Binh’s paper describing substrate-directed, palladium(II)-catalyzed intramolecular hydrofunctionalization has been accepted for publication in Chem. Sci. In this investigation we demonstrate that an bidentate AQ-amide directing group is able to promote otherwise disfavored ring-closure pathways, including those disfavored by Baldwin’s rules (e.g., 5-endo–trig). This marks the culmination of a four-year journey initiated by former high-school intern Jessica (now at MIT) and her mentor John (now at Bristol Myers Squibb). Congratulations are in order for all co-authors for getting this to the finish line: Xin, Zi-Qi, John, Jessica, Van, Hui-Qi, Zhen, Zhonglin, and Kin from Scripps Research; Binh and Peng from University of Pittsburgh; and Rong (and Xin) from Nankai University Medical School.
For a link to the paper, click here: https://pubs.rsc.org/en/content/articlelanding/2020/sc/d0sc03409f#!divAbstract
This work was first described in April in a pre-print that was deposited on ChemRxiv: https://chemrxiv.org/articles/Controlling_Cyclization_Pathways_in_Palladium_II_-Catalyzed_Intramolecular_Alkene_Hydrofunctionalization_via_Substrate_Directivity/12090402/1