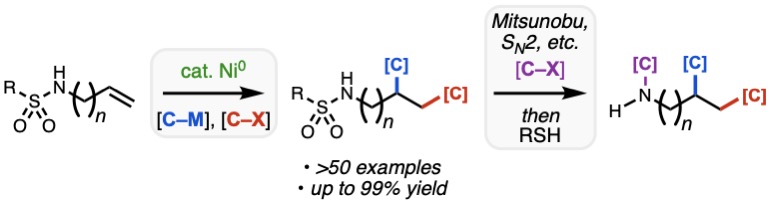
If you need something to “chew” on this Thanksgiving, look no further than our most recent collaborative paper severed by Team Nickel and Bristol Myers Squibb, accepted today for publication in ACS Catalysis. Led by second-year graduate student Omar Apolinar, the manuscript outlines the discovery and development of sulfonamides as uniquely effective nitrogen-based directing groups for the 1,2-diarylation of diverse alkenyl amine derivatives, including those with highly substituted alkenes and those where the alkene is remote from the sulfonamide. By taking advantage of the 4-cyanobenzenesulfonyl group, a close cousin of the venerable nosyl group, the sulfonamide can functional as a dual directing group and masked amine nucleophile, allowing for rapid modular synthesis of highly substituted amine products. Congrats to Omar, Van, Nana, and Joe from our lab and collaborator Mike Schmidt from BMS!
Click here for a link to the paper: https://pubs.acs.org/doi/10.1021/acscatal.0c03857
The work first appeared in pre-print form back in July: https://chemrxiv.org/articles/preprint/Sulfonamide_Directivity_Enables_Ni-Catalyzed_1_2-Diarylation_of_Diverse_Alkenyl_Amines/12642803/1