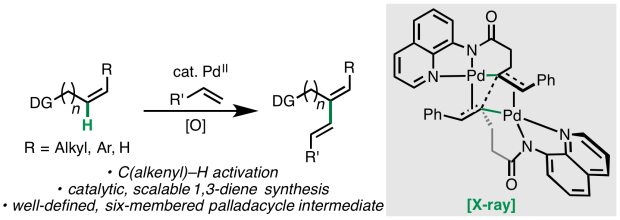
In an Article appearing online today in J. Am. Chem. Soc., the Engle lab and Liu lab (U Pittsburgh) report a detailed study of palladium(II)-catalyzed C(alkenyl)–H activation, including optimization of a method to prepare structurally diverse 1,3-dienes, organometallic synthesis of key intermediates relevant to catalysis, and experimental/computational studies to interrogate the reaction mechanism. Congrats to the whole team: Mingyu, Pusu, and Malkanthi from Scripps; and Yanyan from Pitt. Special shout out to undergraduate co-author Pusu, who was a visiting student from Nankai university for six months. Thanks to the Liu lab for another fruitful collaboration! Way to go, everyone! Click here for a link to the paper.









 (not pictured: Yiyang (Elaine) and Mingyu)
(not pictured: Yiyang (Elaine) and Mingyu)
















 (not pictured: Hui-Qi)
(not pictured: Hui-Qi)