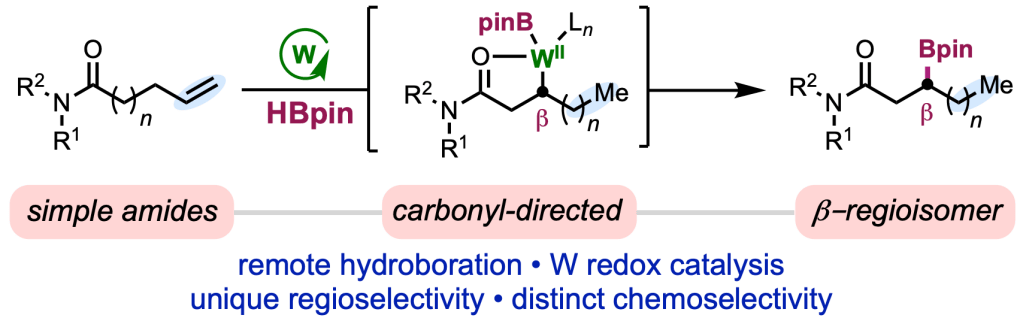
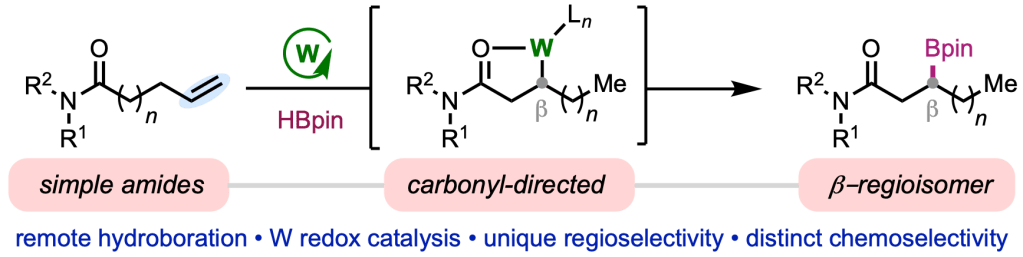
Appearing online today in J. Am. Chem. Soc., we are excited to share the final version of our paper on tungsten-catalyzed alkene isomerization–hydroboration, which we carried out in collaboration with the lab of Prof. Ruben Martin at ICIQ. This project represents an important step forward for our research program in low-valent tungsten-catalyzed alkene functionalization, a largely unexplored family of reactions. In this report, Tanner and Raul teamed up to demonstrate that simple amides are competent directing groups to mediate controlled isomerization and hydroboration of alkenes, allowing functionalization positions that are classical inaccessible via chain-walking methods. The method also represents the first tungsten-catalyzed hydroboration, and the mild nature of the process is exemplified by it’s tolerance for a number of sensitive functional groups (e.g., aryl halides) and epimerizable α-stereocenters. Congrats to Tanner, Raul, and Phillippa, and thanks to the Martin lab for the fun opportunity to join forces!
For a link to the paper in J. Am. Chem. Soc., click here: https://pubs.acs.org/doi/abs/10.1021/jacs.1c07162
As a reminder, a pre-print of the paper first appeared in ChemRxiv back in July: https://chemrxiv.org/engage/chemrxiv/article-details/60e77eb2551cb06ecbadb7a4