This week we are excited to welcome the newest member of the team, Dr. Masahiro Masuda, who joins Scripps Research from Shionogi & Co, Ltd., where he currently works as a medicinal chemist. Dr. Masuda previously earned his Ph.D. in the Department of Applied Biological Chemistry at the University of Tokyo, working in the area of natural products total synthesis with Prof. Hirosato Takikawa. Welcome to the team, Dr. Masuda!
Letian completes his senior-year internship
We bid farewell to Letian Xu who completed his half-year undergrad internship with us this week and is now headed back to Nankai University to write his senior thesis. Letian was a stellar contributor to two different research projects, working under the mentorship of G4 student Juntao Sun. We will miss you and your passion for Bayern Munich, Letian! Can’t wait to see what you accomplish in graduate school and beyond.




C–N Atropisomers via C–H Activation – Now In Press
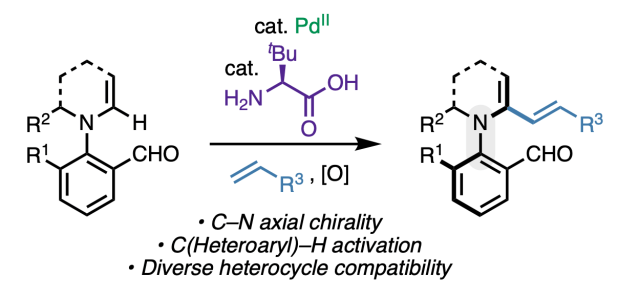
Appearing this week in ACS Catalysis, we report a method to access unique enantioenriched products containing a chiral C–N axis via C(Heteroaryl)–H activation. The reaction relies on the dual catalytic action of palladium(II) and an amino acid, the latter of which acts as chiral transient directing group (cTDG). Through a combination of density functional theory calculations, organometallic studies, and H/D exchange experiments, we showed that the role of the cTDG is to preferentially stabilize one of the two possible regioisomeric organopalladium species formed via C–H activation. Numerous classes of heterocycles are tolerated, and the synthetic utility of the products is demonstrated through various derivatizations, including a diastereoselective [4+2] cycloaddition. Congrats to Juntao, Yiyao, Wenji, Chen-Xi, Shenghua, and Quynh!
For a link to the paper in ACS Catalysis, click here: https://pubs.acs.org/doi/10.1021/acscatal.4c06788
As a reminder, an earlier version of this paper was published as a pre-print in ChemRxiv in November 2023: https://chemrxiv.org/engage/chemrxiv/article-details/6541721bc573f893f1889f75
Copper–Hydride Cluster Paper – Accepted in Angewandte Chemie
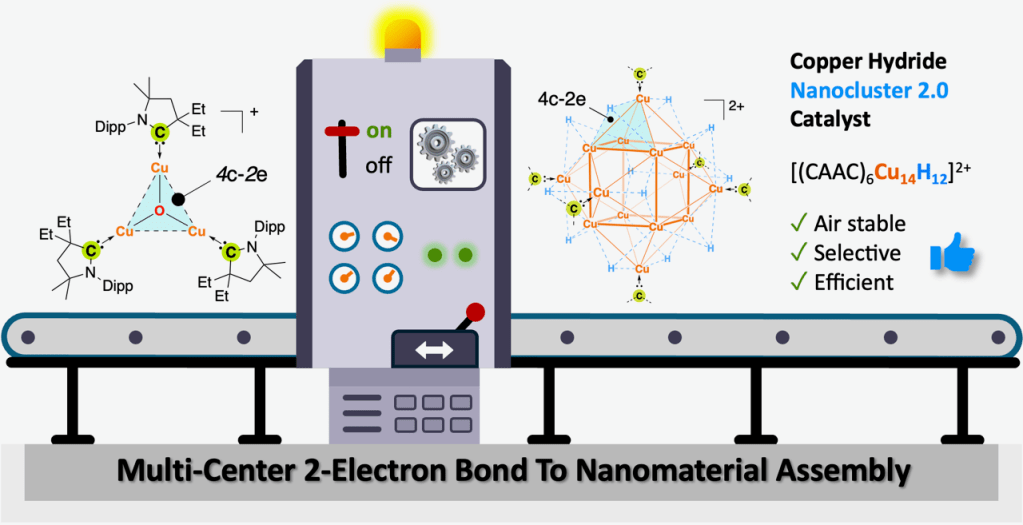
The final peer-reviewed version of our collaborative study with the Payard and Jazzar labs on the synthesis, characterization, and catalytic properties of a novel CAAC-ligated CuH cluster appears this week in Angewandte Chemie. The synthesis of this unique air-stable cluster is enabled by the templating effect of multi-center-2-electron bonds. Akin to Stryker’s reagent, this CuH cluster can mediate conjugate reductions and other useful reactions in organic synthesis. Congrats to the entire team!
For a link to the publication in Angew. Chem. Int. Ed., click here: https://onlinelibrary.wiley.com/doi/10.1002/anie.202419537
As a reminder, an earlier version of this paper was published as a pre-print in ChemRxiv in August 2024: https://chemrxiv.org/engage/chemrxiv/article-details/66c3a7fd20ac769e5f193252
Palladium(COD)(DQ) now available from MilliporeSigma
Pd(COD)(DQ), the stable monometallic Pd(0) precatalyst developed by our group in collaboration with Bristol Myers Squibb has been commercialized by MilliporeSigma and can now be conveniently ordered on a variety of scales (#938718). Try Pd(COD)(DQ) in cross-couplings or other reactions where you would normally turn to Pd2(dba)3. Combine with a phosphine or NHC ligand of choice in situ, add reactants, and you’ll be off to the races.
For a link to the pre-print describing Pd(COD)(DQ), click here: https://chemrxiv.org/engage/chemrxiv/article-details/65e8f798e9ebbb4db918a09f
For a link to the ordering page on MilliporeSigma, click here: https://www.sigmaaldrich.com/US/en/product/aldrich/938718?srsltid=AfmBOooU1-xETxrRkgPu0QwZueJ4SDwMfSpFj_sH6KmVRm_GNa0n4F9F
Comprehensive Bisphosphine Mono-oxide Study – Out this Week in JACS
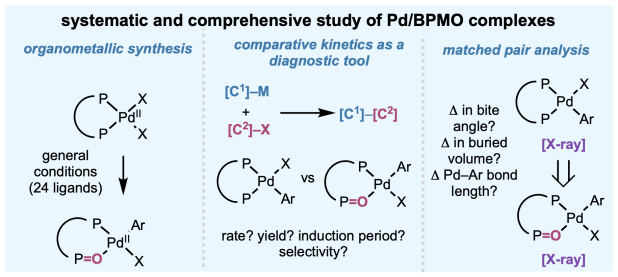
The peer-reviewed version of our paper on palladium–bisphosphine mono-oxide (BPMO) complexes is out this week in J. Am. Chem. Soc. Working in close collaboration with the Blackmond lab and Bristol Myers Squibb over the past three years, we developed a robust method for synthesizing numerous structurally diverse palladium–BPMO oxidative addition complexes bearing different ligands, demonstrate a workflow for diagnosing the relevance of BPMO formation to catalytic reactions of interest, and compare solid state structure of oxidized and non-oxidized “matched pair” complexes to determine the structural consequences of BPMO formation. Congrats to the entire team led by Shenghua!
For a link to the full article in J. Am. Chem. Soc., click here: https://pubs.acs.org/doi/10.1021/jacs.4c10718
As a reminder, the an earlier version of this paper was published as a pre-print in ChemRxiv in August 2024: https://chemrxiv.org/engage/chemrxiv/article-details/66aeabbd5101a2ffa809a6fa
Engle Lab Holiday Party 2024
Ligand-Enabled Regioreversed Conjugate Additions – Pre-print Now Online
In the lab’s latest pre-print, we develop a new and improved catalyst system for “regioreversed” conjugate additions—hydrofunctionalizations that proceed with high levels of α-selectivity owing to the unique mechanism involving a trans-PdII(Ar)(H) intermediate. Key to the success of this study was the discovery that trans-spanning bisphosphine ligands can suppress various undesired side reactions and improve reactivity and selectivity with more challenging alkenyl and alkynyl bromide coupling partners. The method employs our lab’s bench-stable pre-catalysts Pd(COD)(DQ) and operates under air without any special precautions. Congrats to the entire team, Wen-Ji, Evan, Sara, Alena, Gabby, and John!
For a link to the ChemRxiv pre-print, click here: https://chemrxiv.org/engage/chemrxiv/article-details/674696ccf9980725cf181fed
Tungsten-Catalyzed Stereodivergent Olefin Isomierzation – Paper Now In Press
The final peer-reviewed version of our tungsten-catalyzed olefin isomerization method appears in press this week in Chemical Science. The method offers a mild and reliable way to perform kinetic isomerization of β,γ-unsaturated carbonyl compounds without competitive formation of the thermodynamic (conjugated) byproduct. We find that E versus Z stereoselectivity can be controlled by modulating the ligand environment around the tungsten center to grant access to either isomer as desired. Congrats to the project team, Tanner, Camille, Hang Chi (Calvin), and Raul!
For a link to the paper in Chemical Science, click here: https://pubs.rsc.org/en/content/articlelanding/2024/sc/d4sc07093c
As a reminder, an earlier version of this paper was published as a pre-print in ChemRxiv in November 2023: https://chemrxiv.org/engage/chemrxiv/article-details/6554cf6adbd7c8b54b514d16
Omar passes his viva
Congrats to Skaggs Oxford Scholar and Engle lab member, Omar Apolinar, who successfully passed his viva (thesis defense) at Oxford to complete his program, simultaneously earning a Ph.D. in Chemistry from Scripps Research and a D.Phil. in Biochemistry from the University of Oxford. In the Engle’s lab, Omar expanded the scope of native directing groups in nickel-catalyzed olefin diarylation and developed the first enantioselective three-component diarylation of unactivated terminal and internal olefins. At Oxford, Omar performed research under the joint mentorship of Profs. Simon Aldridge and Véronique Gouverneur, developing novel Ca–F complex for nucleophilic fluoride delivery. Congrats Dr. Dr. Apolinar!