Visiting graduate student Xin joins the lab this week. She is doing her graduate studies in medicinal chemistry and will be doing a year long visit in the Engle Lab. Xin is looking forward to enjoying the southern California summer learning more about catalysis.
CONGRATS TO THE CLASS OF 2018!
Huge congratulations to the Class of 2018! The Engle lab is incredibly proud of our recent college and high school graduates, who are moving on to incredible graduate and undergraduate programs around the world!
Undergrads/Visiting Undergrads
Vincent van der Puyl (UCSD): The Scripps Research Institute (Ph.D. in Chemistry)
Yiyang (Elaine) Xiao (Nankai U): Peking U (Ph.D. in Chemistry)
Pusu Yang (Nankai U): SIOC (Ph.D. in Chemistry)
Tian (May) Zeng (UCSD): Caltech (Ph.D. in Chemistry)
High School Student
Forrest Graham (Valhalla High School): Caltech
New summer undergraduate Tim Joins the Lab
Tim Gallagher joins The Engle Lab (mentor Van) for as summer research intern where he hopes to hone in his organometallics knowledge and enjoy the beautiful beaches of southern California. Tim is originally from northern Vermont where he enjoys skiing and snowboarding and will be a 4th year student at Claremont Mckenna College this fall (Wenzel Lab).
Van advances to candidacy
New Review on Conjunctive Cross-Coupling
Congrats to Joe, Van, and Vincent for publication of their invited review on conjunctive cross-coupling which appears online today in Aldrichimica Acta! The review focuses on advances in the use of carbon–carbon π-bonds as conjuctive reagents, or synthetic lynchpins, to merge two conventional reactive fragments under transition metal catalysis. For a link to the article, click here. Way to go team!


Directed 1,2-Dialkylation of Alkenes – Now In Press
Our paper describing directed 1,2-dialkylation of alkenyl carbonyl compounds via nickel catalysis is now online in Chem. Sci. This work represents the first method for controlled introduction of differentiated alkyl groups onto an non-conjugated alkene and offers exciting potential for generating densely functionalized products with high-sp3 content in a rapid manner. Congrats to the authors, Joe, Vincent, Van, and Mingyu! Special shout out to undergraduate co-author and TSRI-SURF program alum Vincent. Click here for a link to the paper. A pre-print of this work was published on ChemRxiv back in January (click here).
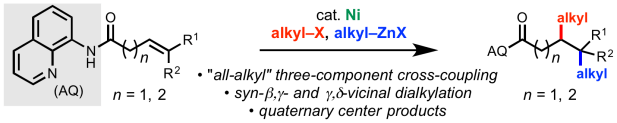

New Postdoc Yang Joins the Lab
Our new postdoc Yang Gao joins us from the Goldman Lab in Rutgers. We’re excited to have another great organometallic chemist join the group and teach us more about Iridium chemistry!
New Undergrad joins the Lab
New undergrad Ryan joins us from UCSD for his first position in a research lab. Ryan has already started working in the hood and is running columns under mentorship of Jose. Welcome to the lab Ryan!
Gamma-Selective Alkene Hydroarylation with Arylboronic Acids
As part of an ongoing collaboration with Pfizer, today we report a new method for achieving γ-selective hydroarylation of alkenyl carbonyl compounds, which is available as a pre-print on ChemRxiv (click here). This reaction is notable for its simplicity, practicality, and functional group tolerance. Because of the ubiquity of arylboronic acids in pharma and in academia, this protocol offers a powerful approach to appending an aryl group of one’s choosing to the γ-position of a carboxylic acid substrate. Congrats to all of the authors: Rei, Tanner, Kin, and Rohan from TSRI; and Gary, Shouliang, Mingying, Fen, and Indra from Pfizer. It was great working with the Pfizer team to field-test the method and to identify valuable heterarylboronic acids that would be of interest to medicinal chemists. Special shoutout to high school student co-author, Rohan, who worked with us earlier this year as part of his junior year internship. Great work, team! Stay tuned for the peer-reviewed version.

San Marcos Bridges Program Lab Visit
Undergraduate students from the San Marcos Bridges Program visited the Engle Lab to get glimpse into the typical work for PhD students in the sciences. Students listened to a lecture by Keary, then participated in a lab tour andtried setting up some air free chemistry in the glovebox. Thanks to the participating lab members for organizing the visit and showing the students the lab!













