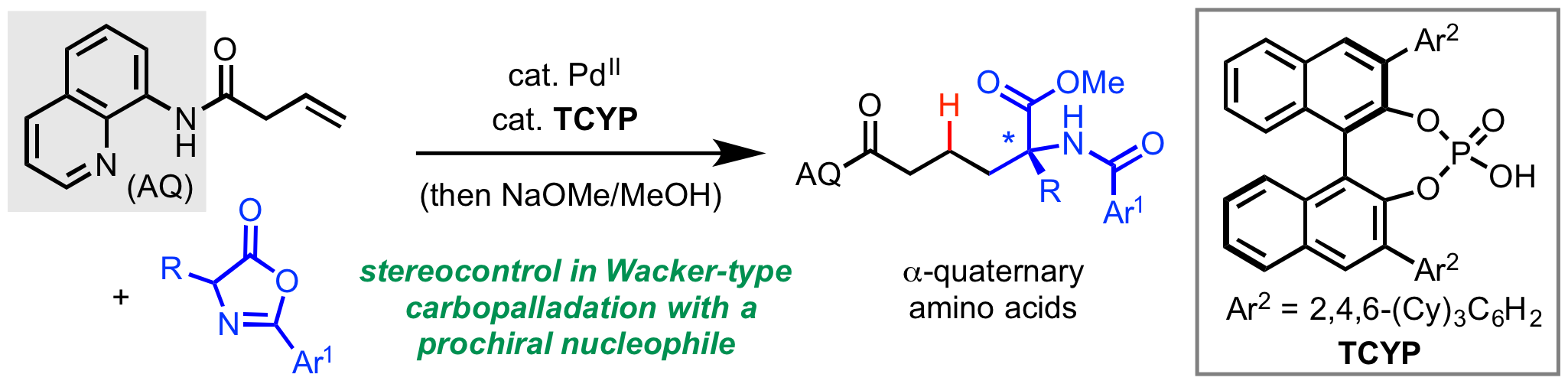
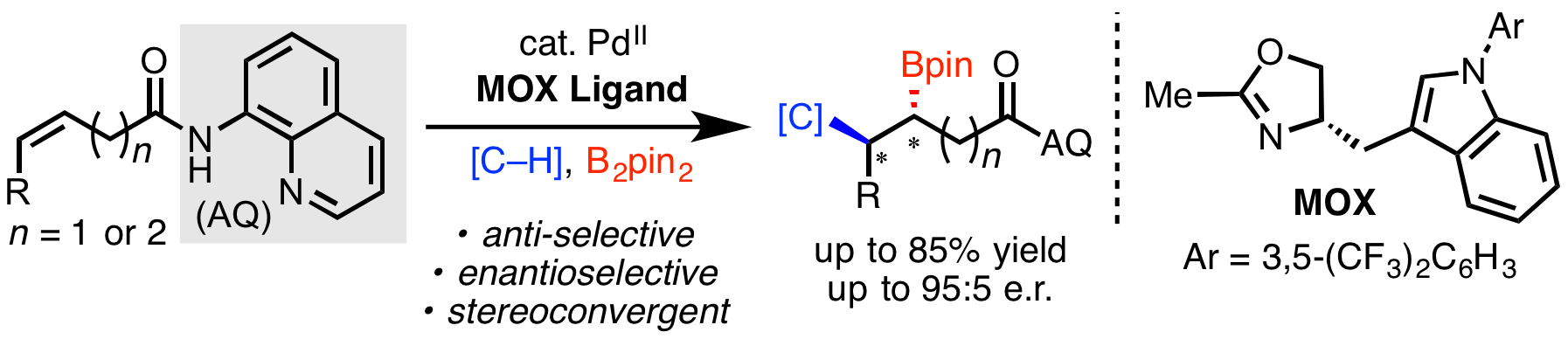
Over the past two years our lab and other labs around the world have been working hard to develop strategies for controlling enantioselectivity in palladium-catalyzed directed hydrofunctionalization and 1,2-difunctionalization reactions. Last month, we described a Pd(II)/chiral phosphoric acid system for enantioselective hydrofunctionalization with prochiral nucleophiles (click here). In a manuscript appearing online today in ACS Catalysis, we describe a new method for enantioselective anti-carboboration, representing the first time that a 1,2-difunctionalization within this family of reactions has been rendered enantioselectivity (click here). We also propose a new mechanistic model based on stoichiometric experiments to account for the stereoconvergent nature of the transformation. Congrats to all of the authors, Zhen, Xiaohan, and Tian (May). Special props to our two undergrad co-authors, Xiaohan and May! In case anyone missed it, a pre-print of this work was published on ChemRxiv back in early January (click here).