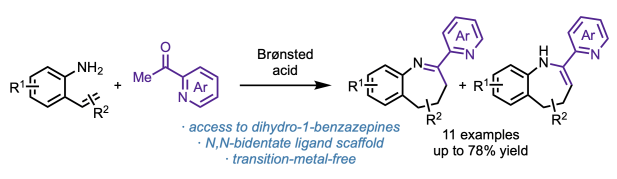
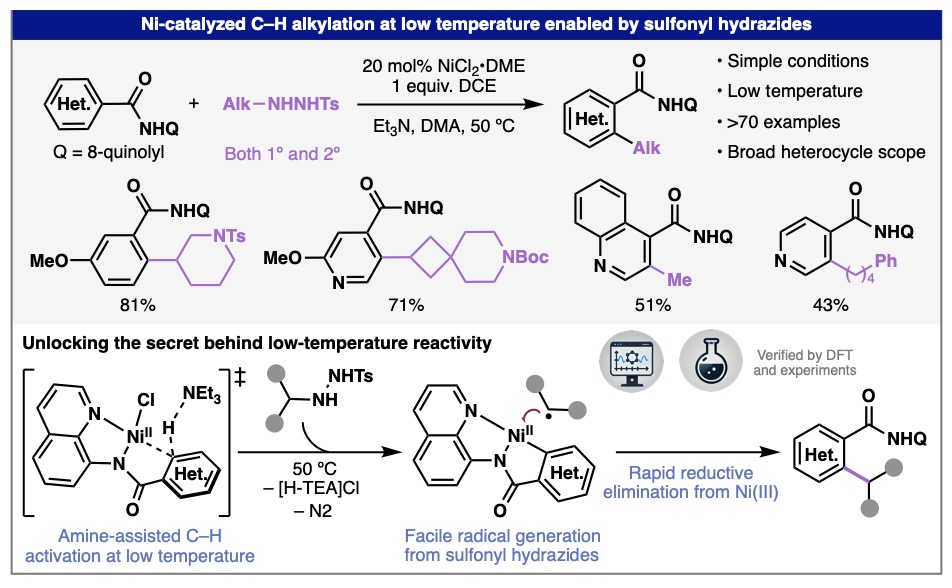
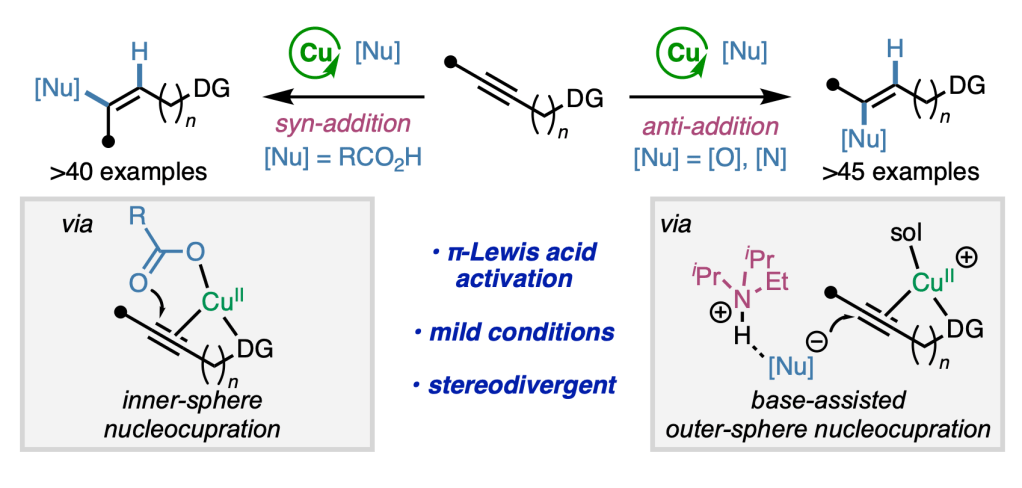
After a fantastic six weeks, we bid farewell to our high school intern Mayia Wagner, who heads back home to complete her senior year at Montrose High School in Colorado. In the Engle lab, Maiya developed new synthetic methods for accessing materials and medicines. Thanks to the Pinhead Institute for supporting this internship program and to Madison for great mentorship throughout the summer!
For a link to Maiya’s blog tracker her summer experience, click here: https://www.pinheadinstitute.org/category/intern-blogs/2025-interns/maiya-wagner/