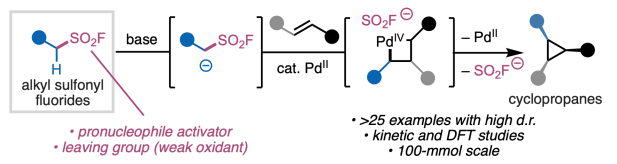
The final peer-reviewed version of our collaborative study on palladium(II)-catalyzed olefin cyclopropanation with alkyl–SO2F compounds appears this week in Nature Synthesis. Largely unexplored in catalysis, alkyl–SO2F compounds are an intriguing class of ambiphiles that can engage in nucleophilic reactivity upon deprotonation and electrophilic reactivity owing the the presence of the -SO2F leaving group. As we discovered in this study, these properties enable alkyl–SO2F to promote cyclopropanation through a unique mechanism that grants access to challenging substitution patterns and stereochemical outcomes. Congrats to the entire team of colleagues from Scripps Research, University of Pittsburgh, Enamine, and Pfizer!
For a link to the paper in Nature Synthesis, click here: https://www.nature.com/articles/s44160-025-00925-1.
As a reminder, we first deposited a pre-print on ChemRxiv back in November, 2024: https://chemrxiv.org/engage/chemrxiv/article-details/672b9b3cf9980725cf547804