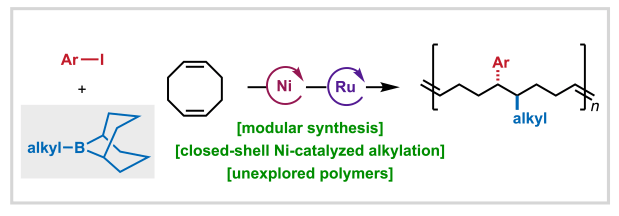
Our lab, together with collaborators at Bristol Myers Squibb, University of Pittsburgh, and Georgia Tech, has developed a new nickel-catalyzed 1,2-alkylarylation of 1,5-cyclooctadiene (COD) that enables access to previously inaccessible cyclooctene monomers bearing C(sp³) and C(sp²) substituents. This method employs alkyl-9-BBN reagents as effective transmetalating partners, maintaining a two-electron redox manifold and avoiding radical pathways incompatible with 1,5-cyclooctadiene. The resulting monomers undergo ring-opening metathesis polymerization (ROMP) to afford new polymers with tunable properties dictated by the alkyl and aryl coupling partners. DFT studies reveal that the enhanced reactivity of alkyl-9-BBN arises from destabilization of the pre-transmetalation complex, distinguishing it from less reactive alkylboronic esters. Congrats to the entire team: Anne, Aimee, and Camille from Scripps Research; Shijia from the Liu lab at the University of Pittsburgh; and Ethan from the Gutekunst lab at Georgia Tech; and Steve from BMS.
For a link to the pre-print in ChemRxiv, click here: https://chemrxiv.org/engage/chemrxiv/article-details/68e901addfd0d042d1dace4c