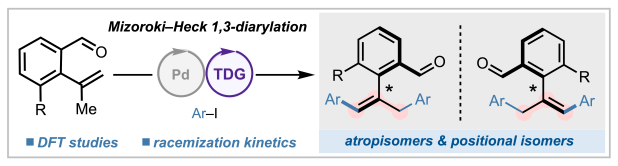
Appearing this week in J. Am. Chem. Soc., we describe a special class of substituted styrenes that exhibit equivalent atrop- and positional isomerism. In other words, inversion about the hindered C(aryl)–C(alkenyl) bond is equivalent to relocating the olefin to adjacent position. These unique molecules can be conveniently accessed via Mizoroki–Heck 1,3-homodiarylation under co-catalysis by palladium and an amino acid transient directing group (TDG), with density functional theory (DFT) calculations shedding light on how the amino acid controls multiple layers of selectivity in this cascade process. Congrats to the entire collaborative team: Amit, Yiyao, Wen-Ji, and Madeline from Scripps Research; and Turki from the Liu lab at the University of Pittsburgh!
For a link to the paper in J. Am. Chem. Soc., click here: https://pubs.acs.org/doi/full/10.1021/jacs.5c10118
As a reminder, we first deposited a pre-print on ChemRxiv back in May; click here for a link: https://chemrxiv.org/engage/chemrxiv/article-details/6835b62d1a8f9bdab53f41a4