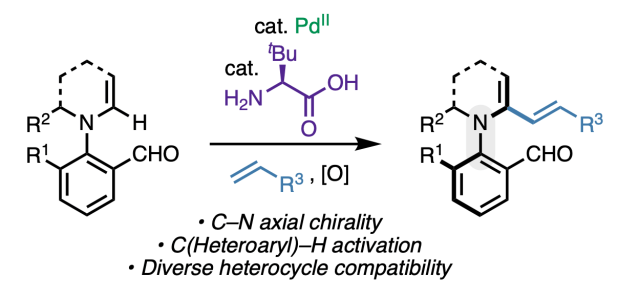
Appearing this week in ACS Catalysis, we report a method to access unique enantioenriched products containing a chiral C–N axis via C(Heteroaryl)–H activation. The reaction relies on the dual catalytic action of palladium(II) and an amino acid, the latter of which acts as chiral transient directing group (cTDG). Through a combination of density functional theory calculations, organometallic studies, and H/D exchange experiments, we showed that the role of the cTDG is to preferentially stabilize one of the two possible regioisomeric organopalladium species formed via C–H activation. Numerous classes of heterocycles are tolerated, and the synthetic utility of the products is demonstrated through various derivatizations, including a diastereoselective [4+2] cycloaddition. Congrats to Juntao, Yiyao, Wenji, Chen-Xi, Shenghua, and Quynh!
For a link to the paper in ACS Catalysis, click here: https://pubs.acs.org/doi/10.1021/acscatal.4c06788
As a reminder, an earlier version of this paper was published as a pre-print in ChemRxiv in November 2023: https://chemrxiv.org/engage/chemrxiv/article-details/6541721bc573f893f1889f75