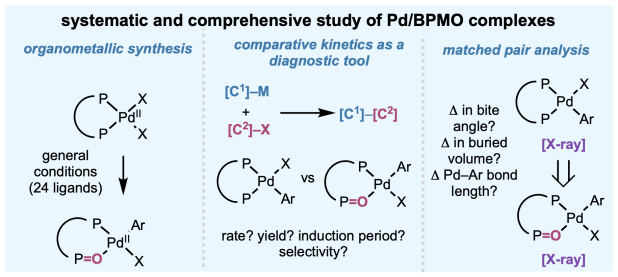
The peer-reviewed version of our paper on palladium–bisphosphine mono-oxide (BPMO) complexes is out this week in J. Am. Chem. Soc. Working in close collaboration with the Blackmond lab and Bristol Myers Squibb over the past three years, we developed a robust method for synthesizing numerous structurally diverse palladium–BPMO oxidative addition complexes bearing different ligands, demonstrate a workflow for diagnosing the relevance of BPMO formation to catalytic reactions of interest, and compare solid state structure of oxidized and non-oxidized “matched pair” complexes to determine the structural consequences of BPMO formation. Congrats to the entire team led by Shenghua!
For a link to the full article in J. Am. Chem. Soc., click here: https://pubs.acs.org/doi/10.1021/jacs.4c10718
As a reminder, the an earlier version of this paper was published as a pre-print in ChemRxiv in August 2024: https://chemrxiv.org/engage/chemrxiv/article-details/66aeabbd5101a2ffa809a6fa