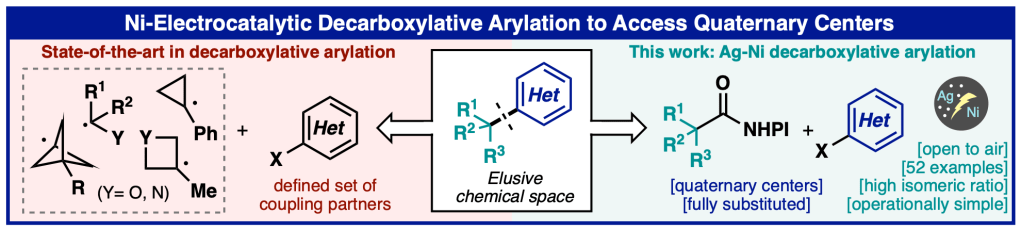
Today, our collaborative study with the Baran lab and colleagues from Bristol Myers Squibb, Biogen, Leo Pharma, and Enamine describing a new nickel/electrocatalytic decarboxylative arylation method to access quaternary centers appeared online in Angewandte Chemie, International Edition. The method uses a unique combination of pyridine and BINAP as ligands to enable an otherwise challenging coupling event. Camille from our lab contributed to elucidating the complex mechanism of this process. We had a blast collaborating with the team on this creative and useful method!
For a link to the paper in Angewandte Chemie, International Edition, click here: https://onlinelibrary.wiley.com/doi/10.1002/anie.202314617
As a reminder, a ChemRxiv pre-print on this work was uploaded in September 2023: https://chemrxiv.org/engage/chemrxiv/article-details/64f248dddd1a73847ffb6e0d
