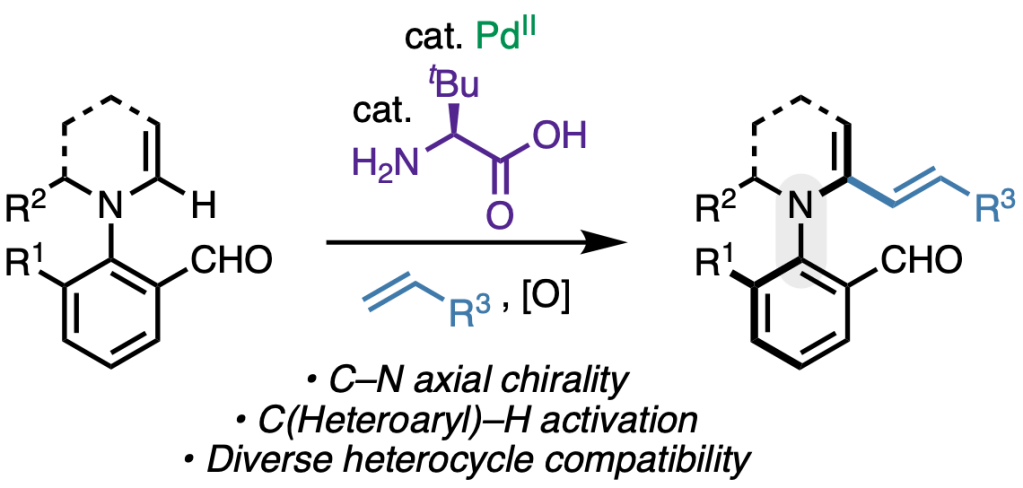In our latest ChemRxiv pre-print we describe a unique atroposelective C(Heteroaryl)–H olefination process the establishes a chiral C–N axis under dual catalysis of Pd(II) and an amino acid transient directing group. Included in the scope are four classes of “enamine-type” azaheterocycle, which all proceed with high levels of enantioselectivity. The diene-type character of the C–H olefinated heterocycles can be leveraged in downstream Diels–Alder [4+2] cycloaddition, granting access to densely functionalized sp3-rich C–N atropisomers. Congrats to the team on this advancement!
For a link to the pre-print in ChemRxiv, click here: https://chemrxiv.org/engage/chemrxiv/article-details/6541721bc573f893f1889f75